Question:

Updated On: Jul 19, 2024
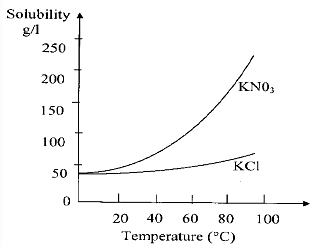
- (A) At room temperature the solubility of and are not equal.
- (B) The solubilities of both and increase with temperature.
- (C) The solubility of KCl decreases with temperature.
- (D) The solubility of increases much more compared to that of with increase in temperature.
Hide Solution
Verified By Collegedunia
The Correct Option is C
Solution and Explanation
Explanation:
We can see from the curve of both the compounds:Solubility increases as temperature increase with different rate. Therefore, statement in option (C) is incorrect as it says solubility decreases with increase in temperature.Hence, the correct option is (C).
We can see from the curve of both the compounds:Solubility increases as temperature increase with different rate. Therefore, statement in option (C) is incorrect as it says solubility decreases with increase in temperature.Hence, the correct option is (C).
Was this answer helpful?
0
0
Top Questions on Bohr’s Model for Hydrogen Atom
- Consider an electron in the $ n = 3 $ orbit of a hydrogen-like atom with atomic number $ Z $. At absolute temperature $ T $, a neutron having thermal energy $ k_B T $ has the same de Broglie wavelength as that of this electron. If this temperature is given by $$ T = \frac{Z^2 h^2}{\alpha \pi^2 a_0^2 m_N k_B} $$ (where $ h $ is Planck’s constant, $ k_B $ is Boltzmann’s constant, $ m_N $ is the mass of the neutron, and $ a_0 $ is the Bohr radius), then the value of $ \alpha $ is ___.
- JEE Advanced - 2025
- Physics
- Bohr’s Model for Hydrogen Atom
- The wavelength of the first line in the Balmer series of hydrogen spectrum is 656 nm. What is the wavelength of the second line?
- CUET (UG) - 2025
- Chemistry
- Bohr’s Model for Hydrogen Atom
- According to Bohr's model of hydrogen atom, which of the following statement is incorrect?
- JEE Main - 2025
- Chemistry
- Bohr’s Model for Hydrogen Atom
Given below are two statements:
Statement (I) : The dimensions of Planck’s constant and angular momentum are same.
Statement (II) : In Bohr’s model, electron revolves around the nucleus in those orbits for which angular momentum is an integral multiple of Planck’s constant.
In the light of the above statements, choose the most appropriate answer from the options given below:- JEE Main - 2025
- Physics
- Bohr’s Model for Hydrogen Atom
- If \(a_0\) is denoted as the Bohr radius of the hydrogen atom, then what is the de-Broglie wavelength \( \lambda \) of the electron present in the second orbit of the hydrogen atom?
- JEE Main - 2025
- Chemistry
- Bohr’s Model for Hydrogen Atom
View More Questions
Questions Asked in MHT CET exam
Which part of root absorb mineral?
- MHT CET - 2025
- The Root
- A body of mass 2 kg is moving in a circular path of radius 3 m with a constant speed of 6 m/s. What is the centripetal force acting on the body?
- MHT CET - 2025
- Centripetal forces
- A 200 g sample of water at 80°C is mixed with 100 g of water at 20°C. Assuming no heat loss to the surroundings, what is the final temperature of the mixture?
- MHT CET - 2025
- thermal properties of matter
- Given the equation: \[ 81 \sin^2 x + 81 \cos^2 x = 30 \] Find the value of \( x \).
- MHT CET - 2025
- Trigonometric Identities
- A body of mass 10 kg is at a height of 5 m above the surface of the Earth. What is the gravitational potential energy of the body? (Take \( g = 10 \, \text{m/s}^2 \))
- MHT CET - 2025
- Gravitational Potential Energy
View More Questions