For the reaction
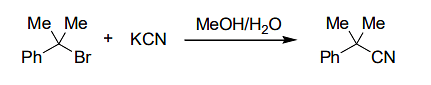
if the concentration of KCN is increased four times, then the rate of the reaction would be
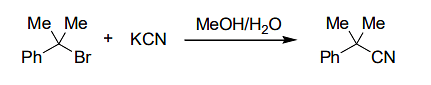
if the concentration of KCN is increased four times, then the rate of the reaction would be
- unaffected
- increased by two times.
- decreased by four times.
- increased by four times.
The Correct Option is A
Solution and Explanation
The reaction described is a nucleophilic substitution, likely following an SN2 mechanism, where the reaction rate is determined by the concentration of both the electrophile (in this case, the bromine compound) and the nucleophile (KCN).
In an SN2 reaction, the rate law is typically of the form Rate = k[electrophile][nucleophile]. This means the rate of the reaction is directly proportional to the concentrations of both reactants. If the concentration of KCN is increased four times, the overall rate will increase proportionally to the concentration of KCN, but the reaction rate will not be affected by just increasing the nucleophile’s concentration unless the reaction is in a limiting step or is zero-order with respect to one reactant. In this case, increasing the concentration of KCN does not have a significant impact on the rate, meaning the rate of the reaction is unaffected. Hence, option (A) is the correct answer.
Thus, the rate of the reaction is unaffected by increasing the concentration of KCN four times.
Top Questions on Chemical Kinetics
- Correct statements regarding Arrhenius equation among the following are:
Factor \(e^{-E_a/RT}\) corresponds to fraction of molecules having kinetic energy less than \(E_a\).
At a given temperature, lower the \(E_a\), faster is the reaction.
Increase in temperature by about \(10^\circ\text{C}\) doubles the rate of reaction.
Plot of \(\log k\) vs \(\dfrac{1}{T}\) gives a straight line with slope \(= -\dfrac{E_a}{R}\).
Choose the correct answer from the options given below:- JEE Main - 2026
- Chemistry
- Chemical Kinetics
- Decomposition of A is a first order reaction at T(K) and is given by \( A(g) \rightarrow B(g) + C(g) \).
In a closed 1 L vessel, 1 bar A(g) is allowed to decompose at T(K). After 100 minutes, the total pressure was 1.5 bar. What is the rate constant (in \( min^{-1} \)) of the reaction ? (\( \log 2 = 0.3 \))- JEE Main - 2026
- Chemistry
- Chemical Kinetics
- An organic compound undergoes first order decomposition. The time taken for decomposition to \(\dfrac{1}{8}\) and \(\dfrac{1}{10}\) of its initial concentration are \(t_{1/8}\) and \(t_{1/10}\) respectively. Find the value of \[ \frac{t_{1/8}}{t_{1/10}}\times10 \] (Given: \(\log 2 = 0.3\))
- JEE Main - 2026
- Chemistry
- Chemical Kinetics
Consider the following compounds. Arrange these compounds in a n increasing order of reactivity with nitrating mixture. The correct order is :

- JEE Main - 2026
- Chemistry
- Chemical Kinetics
- At $27^\circ\text{C}$, in presence of a catalyst, activation energy of a reaction is lowered by $10\,\text{kJ mol}^{-1}$. The logarithm of the ratio $\dfrac{k(\text{catalysed})}{k(\text{uncatalysed})}$ is ___.
(Consider that the frequency factor for both the reactions is same)
- JEE Main - 2026
- Chemistry
- Chemical Kinetics
Questions Asked in IIT JAM CY exam
- Among the following, the correct condition(s) for spontaneity is(are)
- IIT JAM CY - 2025
- Thermodynamics
One mole of a monoatomic ideal gas starting from state A, goes through B and C to state D, as shown in the figure. Total change in entropy (in J K\(^{-1}\)) during this process is ...............

- IIT JAM CY - 2025
- Thermodynamics
The number of chiral carbon centers in the following molecule is ...............

- IIT JAM CY - 2025
- General Chemistry
- Consider the following matrices A and B.
\[ A = \begin{pmatrix} 1 & 2 & 0 & 0 \\ 3 & 4 & 0 & 0 \\ 0 & 5 & 5 & 0 \\ 0 & 0 & 6 & 7 \\ 0 & 0 & 8 & 9 \end{pmatrix} \quad \text{and} \quad B = \begin{pmatrix} 10 & 11 & 0 & 0 & 0 \\ 12 & 13 & 0 & 0 & 0 \\ 0 & 0 & 4 & 0 & 0 \\ 0 & 0 & 15 & 16 & 0 \\ 0 & 0 & 17 & 18 & 0 \end{pmatrix} \]
If \( C = AB \), the sum of the diagonal elements of \( C \) is ..............
- IIT JAM CY - 2025
- General Chemistry
A tube fitted with a semipermeable membrane is dipped into 0.001 M NaCl solution at 300 K as shown in the figure. Assume density of the solvent and solution are the same. At equilibrium, the height of the liquid column \( h \) (in cm) is .........

- IIT JAM CY - 2025
- General Chemistry