Exhaustive hydrogenation of the following compound
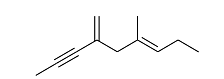
under Pd/C generates a saturated hydrocarbon as the product.
The number of stereoisomers possible for this product is _______.

under Pd/C generates a saturated hydrocarbon as the product.
The number of stereoisomers possible for this product is _______.
Correct Answer: 3
Solution and Explanation
The given compound is an unsaturated hydrocarbon containing two double bonds. Upon exhaustive hydrogenation, both double bonds will be reduced, converting the compound into a saturated hydrocarbon.
The structure of the saturated hydrocarbon will have two stereogenic centers, which will give rise to 3 possible stereoisomers. These include:
- One meso form (achiral due to symmetry).
- Two enantiomers (which are non-superimposable mirror images of each other).
Thus, the number of stereoisomers possible for this product is 3
Top Questions on Stereochemistry
- The heat of atomisation of methane and ethane are \( x \) kJ mol\(^{-1}\) and \( y \) kJ mol\(^{-1}\) respectively. The longest wavelength (\( \lambda \)) of light capable of breaking the C–C bond can be expressed in SI unit as:
- JEE Main - 2026
- Chemistry
- Stereochemistry
- Heat of atomisation of CH$_4$(g) and C$_2$H$_6$(g) are $x$ kJ/mol and $y$ kJ/mol respectively. Find the maximum wavelength of photon required to dissociate C–C bond in C$_2$H$_6$.
- JEE Main - 2026
- Chemistry
- Stereochemistry
Which of the following is true for the stereochemical relationship of the given structures (A-D)?

- CUET (PG) - 2025
- Chemistry
- Stereochemistry
Consider the following molecule (X).
The Structure X is?

- JEE Main - 2025
- Chemistry
- Stereochemistry
How many different stereoisomers are possible for the given molecule?

- JEE Main - 2025
- Chemistry
- Stereochemistry
Questions Asked in IIT JAM CY exam
- Among the following, the correct condition(s) for spontaneity is(are)
- IIT JAM CY - 2025
- Thermodynamics
One mole of a monoatomic ideal gas starting from state A, goes through B and C to state D, as shown in the figure. Total change in entropy (in J K\(^{-1}\)) during this process is ...............

- IIT JAM CY - 2025
- Thermodynamics
The number of chiral carbon centers in the following molecule is ...............

- IIT JAM CY - 2025
- General Chemistry
- Consider the following matrices A and B.
\[ A = \begin{pmatrix} 1 & 2 & 0 & 0 \\ 3 & 4 & 0 & 0 \\ 0 & 5 & 5 & 0 \\ 0 & 0 & 6 & 7 \\ 0 & 0 & 8 & 9 \end{pmatrix} \quad \text{and} \quad B = \begin{pmatrix} 10 & 11 & 0 & 0 & 0 \\ 12 & 13 & 0 & 0 & 0 \\ 0 & 0 & 4 & 0 & 0 \\ 0 & 0 & 15 & 16 & 0 \\ 0 & 0 & 17 & 18 & 0 \end{pmatrix} \]
If \( C = AB \), the sum of the diagonal elements of \( C \) is ..............
- IIT JAM CY - 2025
- General Chemistry
A tube fitted with a semipermeable membrane is dipped into 0.001 M NaCl solution at 300 K as shown in the figure. Assume density of the solvent and solution are the same. At equilibrium, the height of the liquid column \( h \) (in cm) is .........

- IIT JAM CY - 2025
- General Chemistry