Consider the following molecules: Br3O8, F2O, H2S4O6, H2S5O6, and C3O2. Count the number of atoms existing in their zero oxidation state in each molecule. Their sum is____.
Correct Answer: 6
Approach Solution - 1
Structures and Oxidation States:
The first molecule shows a bromine (Br) atom with oxidation states of +6 and +4, and the oxygen atoms (O) have oxidation states of -1. The fluorine (F) atom has an oxidation state of -1.
The second structure features sulfur (S) atoms with oxidation states of +5. The oxygen atoms (O) in the sulfonic acid groups have a zero oxidation state, while the hydrogen atoms (H) are in their usual +1 state.
The third structure has two carbon (C) atoms each in the +2 oxidation state, with oxygen atoms in zero oxidation states.
Summary of Oxidation States:
- In the first structure, atoms of zero oxidation number: 0.
- In the second structure, the sulfur atoms (S) have +5 oxidation states, and oxygen atoms (O) have 0 oxidation states, totaling 2 atoms with zero oxidation states.
- In the third structure, the carbon atoms (C) each have a +2 oxidation state, while the oxygen atoms (O) are in zero oxidation state, giving 4 atoms with zero oxidation states.
Conclusion: The total number of atoms with zero oxidation state is 6. Thus, the total number of atoms with zero oxidation state in the given structures is 6.
Approach Solution -2
The correct answer is 6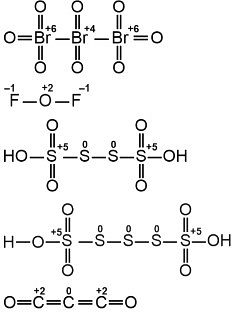
Total atom with zero oxidation number state are 6.
Top Questions on Structure of atom
- Consider the last electron of an element having atomic number $9$ and choose the correct option.
- JEE Main - 2025
- Chemistry
- Structure of atom
- A ligand which has two different donor atoms and either of the two ligates with the central metal atom/ion in the complex is called
- KCET - 2025
- Chemistry
- Structure of atom
- An element 'M' has 25% of the electrons filled in the third shell as in the second shell. The element 'M' is :
- CBSE Class X - 2025
- Science
- Structure of atom
- The energy associated with electron in first orbit of hydrogen atom is \(-2.18 \times 10^{-18}\) J. The frequency of the light required (in Hz) to excite the electron to fifth orbit is (\(h=6.6 \times 10^{-34}\) Js)
- TS EAMCET - 2025
- Chemistry
- Structure of atom
- Isotopes of an element have a different number of
- UPCATET - 2025
- Physics
- Structure of atom
Questions Asked in JEE Advanced exam
- Let $ x_0 $ be the real number such that $ e^{x_0} + x_0 = 0 $. For a given real number $ \alpha $, define $$ g(x) = \frac{3xe^x + 3x - \alpha e^x - \alpha x}{3(e^x + 1)} $$ for all real numbers $ x $. Then which one of the following statements is TRUE?
- JEE Advanced - 2025
- Fundamental Theorem of Calculus
- A linear octasaccharide (molar mass = 1024 g mol$^{-1}$) on complete hydrolysis produces three monosaccharides: ribose, 2-deoxyribose and glucose. The amount of 2-deoxyribose formed is 58.26 % (w/w) of the total amount of the monosaccharides produced in the hydrolyzed products. The number of ribose unit(s) present in one molecule of octasaccharide is _____.
Use: Molar mass (in g mol$^{-1}$): ribose = 150, 2-deoxyribose = 134, glucose = 180; Atomic mass (in amu): H = 1, O = 16- JEE Advanced - 2025
- Biomolecules
Let $ P(x_1, y_1) $ and $ Q(x_2, y_2) $ be two distinct points on the ellipse $$ \frac{x^2}{9} + \frac{y^2}{4} = 1 $$ such that $ y_1 > 0 $, and $ y_2 > 0 $. Let $ C $ denote the circle $ x^2 + y^2 = 9 $, and $ M $ be the point $ (3, 0) $. Suppose the line $ x = x_1 $ intersects $ C $ at $ R $, and the line $ x = x_2 $ intersects $ C $ at $ S $, such that the $ y $-coordinates of $ R $ and $ S $ are positive. Let $ \angle ROM = \frac{\pi}{6} $ and $ \angle SOM = \frac{\pi}{3} $, where $ O $ denotes the origin $ (0, 0) $. Let $ |XY| $ denote the length of the line segment $ XY $. Then which of the following statements is (are) TRUE?
- JEE Advanced - 2025
- Conic sections
- Adsorption of phenol from its aqueous solution on to fly ash obeys Freundlich isotherm. At a given temperature, from 10 mg g$^{-1}$ and 16 mg g$^{-1}$ aqueous phenol solutions, the concentrations of adsorbed phenol are measured to be 4 mg g$^{-1}$ and 10 mg g$^{-1}$, respectively. At this temperature, the concentration (in mg g$^{-1}$) of adsorbed phenol from 20 mg g$^{-1}$ aqueous solution of phenol will be ____. Use: $\log_{10} 2 = 0.3$
- JEE Advanced - 2025
- Adsorption
- At 300 K, an ideal dilute solution of a macromolecule exerts osmotic pressure that is expressed in terms of the height (h) of the solution (density = 1.00 g cm$^{-3}$) where h is equal to 2.00 cm. If the concentration of the dilute solution of the macromolecule is 2.00 g dm$^{-3}$, the molar mass of the macromolecule is calculated to be $X \times 10^{4}$ g mol$^{-1}$. The value of $X$ is ____. Use: Universal gas constant (R) = 8.3 J K$^{-1}$ mol$^{-1}$ and acceleration due to gravity (g) = 10 m s$^{-2}\}$
- JEE Advanced - 2025
- Colligative Properties
Concepts Used:
Atom Structure Models
The three atomic models are as follows:
Thomson model:
Thomson atomic model was proposed by William Thomson in the year 1900. This model explained the description of an inner structure of the atom theoretically. It was strongly supported by Sir Joseph Thomson, who had discovered the electron earlier.
Thomson assumed that an electron is two thousand times lighter than a proton and believed that an atom is made up of thousands of electrons. In this atomic structure model, he considered atoms surrounded by a cloud having positive as well as negative charges. The demonstration of the ionization of air by X-ray was also done by him together with Rutherford. They were the first to demonstrate it. Thomson’s model of an atom is similar to a plum pudding.
Rutherford’s Alpha Scattering Experiment:
Rutherford’s conducted an experiment by bombarding a thin sheet of gold with α-particles and then studied the trajectory of these particles after their interaction with the gold foil.
Bohr’s Model of an Atom:
Bohr model of the atom was proposed by Neil Bohr in 1915. It came into existence with the modification of Rutherford’s model of an atom. Rutherford’s model introduced the nuclear model of an atom, in which he explained that a nucleus (positively charged) is surrounded by negatively charged electrons.