Compare the chemistry of the actinoids with that of lanthanoids with reference to:
- electronic configuration
- oxidation states and
- chemical reactivity.
Compare the chemistry of the actinoids with that of lanthanoids with reference to:
- electronic configuration
- oxidation states and
- chemical reactivity.
Solution and Explanation
Electronic configuration
The general electronic configuration for lanthanoids is [Xe]54 4f0-14 5d0-1 6s2 and that for actinoids is [Rn]86 5f1-14 6d0-1 7s2 . Unlike 4f orbitals, 5f orbitals are not deeply buried and participate in bonding to a greater extent.
Oxidation states
The principal oxidation state of lanthanoids is (+3). However, sometimes we also encounter oxidation states of +2 and +4. This is because of extra stability of fully-filled and half-filled orbitals. Actinoids exhibit a greater range of oxidation states. This is because the 5f, 6d, and 7s levels are of comparable energies. Again, (+3) is the principal oxidation state for actinoids. Actinoids such as lanthanoids have more compounds in +3 state than in +4 state.
Chemical reactivity
In the lanthanide series, the earlier members of the series are more reactive. They have reactivity that is comparable to Ca. With an increase in the atomic number, the lanthanides start behaving similar to Al. Actinoids, on the other hand, are highly reactive metals, especially when they are finely divided. When they are added to boiling water, they give a mixture of oxide and hydride. Actinoids combine with most of the non-metals at moderate temperatures. Alkalies have no action on these actinoids. In case of acids, they are slightly affected by nitric acid (because of the formation of a protective oxide layer).
Top Questions on d -and f -Block Elements
- At temperature \(T\) K, \(2\) moles of liquid \(A\) and \(3\) moles of liquid \(B\) are mixed. The vapour pressure of the ideal solution formed is \(320\) mm Hg. At this stage, one mole of \(A\) and one mole of \(B\) are added to the solution. The vapour pressure is now measured as \(328.6\) mm Hg. The vapour pressures (in mm Hg) of pure \(A\) and pure \(B\) respectively are:
- JEE Main - 2026
- Chemistry
- d -and f -Block Elements
- Consider the general reaction given below at 400 K: xA(g) ⇌ yB(g). The values of K___p and K___c are studied under the same condition of temperature but variation in x and y.
(i) K___p = 85.87 and K___c = 2.586
(ii) K___p = 0.862 and K___c = 28.62.
The values of x and y in (i) and (ii) respectively are :- JEE Main - 2026
- Chemistry
- d -and f -Block Elements
- Among the following oxides of 3d elements, the number of mixed oxides are ____________.
\[ \mathrm{Ti_2O_3,\ V_2O_4,\ Cr_2O_3,\ Mn_3O_4,\ Fe_3O_4,\ Fe_2O_3,\ Co_3O_4} \]- JEE Main - 2026
- Chemistry
- d -and f -Block Elements
- Statement-I : Consider the following pairs of ions $(\text{Sc}^{3+}, \text{Ti}^{3+}), (\text{Ti}^{4+}, \text{Ni}^{2+}), (\text{Cu}^{2+}, \text{Zn}^{2+})$ and $(\text{Cr}^{3+}, \text{Mn}^{3+})$. Out of these pairs three pairs consist of ions that are both coloured :
Statement-II : Among the lanthanide ions $\text{Eu}^{2+}$, $\text{Gd}^{3+}$, $\text{Ce}^{4+}$ and $\text{Tb}^{4+}$, the ion $\text{Tb}^{4+}$ is the strongest reducing agent.
Choose the correct option.- JEE Main - 2026
- Chemistry
- d -and f -Block Elements
- Why is the ability of oxygen greater than fluorine to stabilise higher oxidation states of transition metals?
- CBSE CLASS XII - 2025
- Chemistry
- d -and f -Block Elements
Questions Asked in CBSE CLASS XII exam
- (a) Briefly explain Einstein’s photoelectric equation.
(b) Four metals with their work functions are listed below:
K = 2.3 eV, Na = 2.75 eV, Mo = 4.17 eV, Ni = 5.15 eV.
The radiation of wavelength 330 nm from a laser source placed 1 m away, falls on these metals.
Which of these metals will not show photoelectric emission?
What will happen if the laser source is brought closer to a distance of 50 cm?- CBSE CLASS XII - 2025
- Photoelectric Effect
- Differentiate $2\cos^2 x$ w.r.t. $\cos^2 x$.
- CBSE CLASS XII - 2025
- Derivatives
- The diagonals of a parallelogram are given by \( \mathbf{a} = 2 \hat{i} - \hat{j} + \hat{k} \) and \( \mathbf{b} = \hat{i} + 3 \hat{j} - \hat{k}\) . Find the area of the parallelogram.
- Altima Ltd. invited applications for 2,00,000 equity shares of ₹ 10 at a premium of ₹ 4 per share. Amount payable:
On application and allotment – ₹ 7 (incl. ₹ 1 premium)
On first and final call – Balance.
Applications received for 2,40,000 shares. 30,000 rejected. Manvi allotted 4,000 shares failed to pay first and final call. Her shares were forfeited. These were reissued at ₹ 4 per share fully paid-up.
Pass journal entries in the books of Altima Ltd.- CBSE CLASS XII - 2025
- Accounting for Share Capital
- Which of the following options shows the correct chronological order of events related to Indian National Movement?
I. Second Round Table Conference
II. Peasant Movement in Bardoli
III. Champaran Satyagraha
IV. Jallianwala Bagh Massacre- CBSE CLASS XII - 2025
- Indian Freedom Movement
Concepts Used:
Lanthanoids
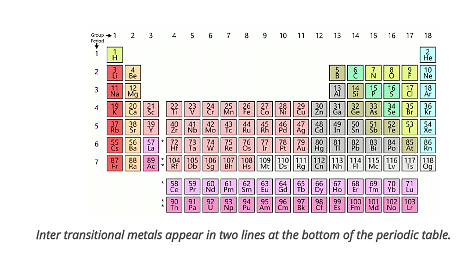
Lanthanoids are at the top of these two-row, while actinoids are at the bottom row.
Properties of Lanthanoids
Lanthanoids are inclusive of 14 elements, with atomic numbers 58-71:
- Cerium - Xe 4f1 5d1 6s2
- Praseodymium - Xe 4f3 6s2
- Neodymium - Xe 4f4 6s2
- Promethium - Xe 4f5 6s2
- Samarium - Xe 4f6 6s2
- Europium - Xe 4f7 6s2
- Gadolinium - Xe 4f7 5d1 6s2
- Terbium - Xe 4f9 6s2
- Dysprosium - Xe 4f10 6s2
- Holmium - Xe 4f11 6s2
- Erbium - Xe 4f12 6s2
- Thulium - Xe 4f13 6s2
- Ytterbium - Xe 4f14 6s2
- Lutetium - Xe 4f14 5d1 6s2
These elements are also called rare earth elements. They are found naturally on the earth, and they're all radioactively stable except promethium, which is radioactive. A trend is one of the interesting properties of the lanthanoid elements, called lanthanide contraction.