Choose the correct and major product formed in the below-given reaction from the choices listed below:
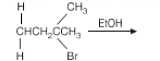
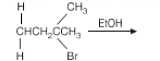
- 3-Pentene
- 2-Methyl-2-butene
- 1-Pentane
- 1-Methyl-2-butene
The Correct Option is B
Solution and Explanation
In the given reaction, we need to determine the major product formed from a β-elimination (dehydrohalogenation) reaction, which typically follows the E2 mechanism.
The given compound is 2-Bromo-3-methylbutane. When reacted with ethanol (EtOH), a protic solvent, the elimination reaction results in the removal of the halogen (Br) and a hydrogen atom from the adjacent carbon atom, forming a double bond.
Applying Zaitsev's rule, which states that the more substituted alkene will be favored as the major product, we look for the formation of a double bond with greater substitution:
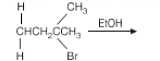
The possible alkenes are:
- 3-Pentene - Less substituted alkene.
- 2-Methyl-2-butene - More substituted alkene.
- 1-Pentene - Least substituted alkene.
- 1-Methyl-2-butene - Less substituted compared to 2-methyl-2-butene.
Thus, according to Zaitsev's rule, 2-Methyl-2-butene will be the major product as it is the more substituted alkene.
The reaction can be represented as follows:
\(\text{2-Bromo-3-methylbutane} \xrightarrow{\text{EtOH}} 2-\text{Methyl-2-butene} + \text{HBr}\)
Therefore, the correct answer is 2-Methyl-2-butene.
Top Questions on carbonyl compounds
Given below are the four isomeric compounds \(P, Q, R, S\):

\(P\): Aromatic compound containing an \(-\mathrm{OH}\) group
\(Q\): Aromatic compound containing an \(-\mathrm{CHO}\) group (aldehyde)
\(R\): Aromatic compound containing a ketone group
\(S\): Aromatic compound containing a ketone group Identify the correct statements from below:
[A.] \(Q, R\) and \(S\) will give precipitate with \(2,4\)-DNP.
[B.] \(P\) and \(Q\) will give positive Baeyer’s test.
[C.] \(Q\) and \(R\) will give sooty flame.
[D.] \(R\) and \(S\) will give yellow precipitate with \(I_2/\mathrm{NaOH}\).
[E.] \(Q\) alone will deposit silver with Tollens’ reagent. Choose the correct option.- JEE Main - 2026
- Chemistry
- carbonyl compounds
- The statements that are incorrect about the nickel(II) complex of dimethylglyoxime are :
A. It is red in colour.
B. It has a high solubility in water at pH = 9.
C. The Ni ion has two unpaired d-electrons.
D. The N – Ni – N bond angle is almost close to 90°.
E. The complex contains four five-membered metallacycles (metal containing rings).
Choose the correct answer from the options given below :- JEE Main - 2026
- Chemistry
- carbonyl compounds
Match the LIST-I with LIST-II

Choose the correct answer from the options given below:- JEE Main - 2026
- Chemistry
- carbonyl compounds
- Which of the following is the correct order with respect to the property indicated?
- JEE Main - 2026
- Chemistry
- carbonyl compounds
- C₆H₁₂O₃ gives positive iodoform test on hydrolysis with dil. Acid. The hydrolysis product formed gives Tollens’ and iodoform test both. Find structure of C₆H₁₂O₃.

- JEE Main - 2026
- Chemistry
- carbonyl compounds
Questions Asked in GPAT exam
- Match the following:
(1) Schedule FF
(2) Schedule F3
(3) Schedule V
(4) Schedule Y
Descriptions:
(P) Standards of patent and proprietary medicines
(Q) Requirements and guidelines for clinical trials
(R) Standards for sterilized umbilical tapes
(S) Standards for Ophthalmic preparations- GPAT - 2025
- Drug therapy
- If the label or the container bears the name of an individual or company purporting to be the manufacturer of the drug, which individual or company is fictitious or does not exist, it is:
- GPAT - 2025
- Drug therapy
- Manufacturing Specification for tooling has been standardized by?
- GPAT - 2025
- Pharmacy Profession & Introduction to Pharmaceuticals
- As per USP, the maximum concentration of benzalkonium chloride used as a preservative in parenteral formulations is
- GPAT - 2025
- Pharmaceutical Analysis
Match the following:
(P) Schedule H
(Q) Schedule G
(R) Schedule P
(S) Schedule F2
Descriptions:
(I) Life period of drugs
(II) Drugs used under RMP
(III) List of Prescription Drugs
(IV) Standards for surgical dressing
- GPAT - 2025
- Drug therapy