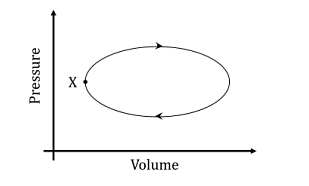
A system undergoes one clockwise cycle from point X back to point X as shown in the figure below:
The correct statement about this process is

The correct statement about this process is
- Internal energy of the system decreases at the end of the cycle
- Entropy of the system increases at the end of the cycle
- System performs work on the surroundings during the cycle.
- Heat exchanged between system and surroundings is zero during the cycle.
The Correct Option is C
Solution and Explanation
In the given cyclic process, the system undergoes a clockwise cycle, indicating that work is being done by the system on the surroundings. During a clockwise cycle on a Pressure-Volume (P-V) diagram, the system expands (increasing volume), which corresponds to the system performing work on the surroundings.
- (A) is false because in a cyclic process, the internal energy of the system remains the same after one complete cycle.
- (B) is false because the total entropy change of the system is zero in a reversible cyclic process.
- (C) is true. The system performs positive work on the surroundings as it expands.
- (D) is false because there is heat exchange between the system and surroundings during a non-adiabatic process.
Top Questions on Thermodynamics
- A weak acid HA has degree of dissociation x. Which option gives the correct expression of \(pH - pK_a\)?
- JEE Main - 2025
- Chemistry
- Thermodynamics
- The molecules having square pyramidal geometry are:
- JEE Main - 2025
- Chemistry
- Thermodynamics
- The standard reduction potential values of some of the p-block ions are given below. Predict the one with the strongest oxidising capacity.
- JEE Main - 2025
- Chemistry
- Thermodynamics
- The incorrect decreasing order of atomic radii is:
- JEE Main - 2025
- Chemistry
- Thermodynamics
- What is the freezing point depression constant of a solvent, 50 g of which contain 1 g non-volatile solute (molar mass 256 g mol\(^{-1}\)) and the decrease in freezing point is 0.40 K?
- JEE Main - 2025
- Chemistry
- Thermodynamics
Questions Asked in IIT JAM CY exam
The number of chiral carbon centers in the following molecule is ...............

- IIT JAM CY - 2025
- General Chemistry
- Consider the following matrices A and B.
\[ A = \begin{pmatrix} 1 & 2 & 0 & 0 \\ 3 & 4 & 0 & 0 \\ 0 & 5 & 5 & 0 \\ 0 & 0 & 6 & 7 \\ 0 & 0 & 8 & 9 \end{pmatrix} \quad \text{and} \quad B = \begin{pmatrix} 10 & 11 & 0 & 0 & 0 \\ 12 & 13 & 0 & 0 & 0 \\ 0 & 0 & 4 & 0 & 0 \\ 0 & 0 & 15 & 16 & 0 \\ 0 & 0 & 17 & 18 & 0 \end{pmatrix} \]
If \( C = AB \), the sum of the diagonal elements of \( C \) is ..............
- IIT JAM CY - 2025
- General Chemistry
A tube fitted with a semipermeable membrane is dipped into 0.001 M NaCl solution at 300 K as shown in the figure. Assume density of the solvent and solution are the same. At equilibrium, the height of the liquid column \( h \) (in cm) is .........

- IIT JAM CY - 2025
- General Chemistry
An electron at rest is accelerated through 10 kV potential. The de Broglie wavelength (in A) of the electron is .............
- IIT JAM CY - 2025
- General Chemistry
The number of stereoisomers possible for the following compound is ..............

- IIT JAM CY - 2025
- General Chemistry