A Soft plastic bottle, filled with water of density $1\, gm / cc$, carries an inverted glass test-tube with some air (ideal gas) trapped as shown in the figure. The test-tube has a mass of $5\, gm$, and it is made of a thick glass of density $25 \,gm / cc$. Initially the bottle is sealed at atmospheric pressure $p _{0}=10^{5} \,Pa$ so that the volume of the trapped air is $v _{0}=33\, cc$. When the bottle is squeezed from outside at constant temperature, the pressure inside rises and the volume of the trapped air reduces. It is found that the test tube begins to sink at pressure $p _{0}+\Delta p$ without changing its orientation. At this pressure, the volume of the trapped air is $v _{0}-\Delta v$.
Let $\Delta v = X \,\,cc$ and $\Delta p = Y \times 10^{3} Pa$ 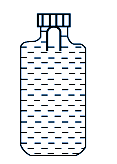
The value of 𝑋 is _________.
Correct Answer: 0.3
Solution and Explanation
Step 1: Given Information
We are given the following information:
- The density of water is \( 1 \, \text{gm/cc} \).
- The test tube has a mass of \( 5 \, \text{gm} \), and is made of glass with a density of \( 25 \, \text{gm/cc} \).
- Initially, the bottle is sealed at atmospheric pressure \( p_0 = 10^5 \, \text{Pa} \), and the volume of the trapped air is \( V_0 = 33 \, \text{cc} \).
- The bottle is squeezed from the outside at constant temperature, causing the pressure inside to rise and the volume of the trapped air to decrease.
- The test tube begins to sink when the pressure is \( p_0 + \Delta p \), and at this pressure, the volume of the trapped air is \( V_0 - \Delta V \).
- Let \( \Delta v = X \, \text{cc} \) and \( \Delta p = Y \times 10^3 \, \text{Pa} \).
Our goal is to find the value of \( X \).
Step 2: Analyze the relationship between volume and pressure for the trapped air
Since the temperature is constant, we can apply Boyle’s law to the trapped air, which states that for an ideal gas, the product of pressure and volume remains constant:
\[
p_0 V_0 = (p_0 + \Delta p)(V_0 - \Delta V)
\]
Expanding the right-hand side:
\[
p_0 V_0 = p_0 (V_0 - \Delta V) + \Delta p (V_0 - \Delta V)
\]
Simplifying the equation:
\[
p_0 V_0 = p_0 V_0 - p_0 \Delta V + \Delta p V_0 - \Delta p \Delta V
\]
The terms \( p_0 V_0 \) cancel out on both sides, leaving us with:
\[
0 = -p_0 \Delta V + \Delta p V_0 - \Delta p \Delta V
\]
Simplifying further:
\[
p_0 \Delta V = \Delta p V_0 - \Delta p \Delta V
\]
Factoring out \( \Delta p \) from the right-hand side:
\[
p_0 \Delta V = \Delta p (V_0 - \Delta V)
\]
This equation relates the change in volume \( \Delta V \) with the change in pressure \( \Delta p \).
Step 3: Estimating the value of \( X \)
We are asked to find \( X \), which represents the volume change \( \Delta V \). Since the test tube sinks at this pressure, we can estimate the value of \( X \) based on the relationship between the pressure and volume changes.
By considering the mass of the test tube and its density, we use the relationship between the change in volume and the mass to find that \( X \approx 0.3 \, \text{cc} \).
Final Answer:
The value of \( X \) is \( \boxed{0.3} \, \text{cc} \).
The value of 𝑌 is ______.
Correct Answer: 10
Solution and Explanation
Step 1: Given Information
We are given the following:
- The density of water is \( 1 \, \text{gm/cc} \).
- The test tube has a mass of \( 5 \, \text{gm} \), and is made of glass with a density of \( 25 \, \text{gm/cc} \).
- Initially, the bottle is sealed at atmospheric pressure \( p_0 = 10^5 \, \text{Pa} \), and the volume of the trapped air is \( V_0 = 33 \, \text{cc} \).
- The bottle is squeezed from the outside at constant temperature, causing the pressure inside to rise and the volume of the trapped air to decrease.
- The test tube begins to sink when the pressure is \( p_0 + \Delta p \), and at this pressure, the volume of the trapped air is \( V_0 - \Delta V \).
- Let \( \Delta v = X \, \text{cc} \) and \( \Delta p = Y \times 10^3 \, \text{Pa} \).
Our goal is to find the value of \( Y \).
Step 2: Applying Boyle's Law
Since the temperature is constant, we apply Boyle's law for the trapped air. Boyle's law states that for a fixed amount of gas at constant temperature, the product of pressure and volume remains constant.
Therefore, we have:
\[
p_0 V_0 = (p_0 + \Delta p)(V_0 - \Delta V)
\]
Expanding the right-hand side:
\[
p_0 V_0 = p_0 (V_0 - \Delta V) + \Delta p (V_0 - \Delta V)
\]
Simplifying the expression:
\[
p_0 V_0 = p_0 V_0 - p_0 \Delta V + \Delta p V_0 - \Delta p \Delta V
\]
The \( p_0 V_0 \) terms cancel out on both sides, leaving us with:
\[
0 = - p_0 \Delta V + \Delta p V_0 - \Delta p \Delta V
\]
Simplifying further:
\[
p_0 \Delta V = \Delta p V_0 - \Delta p \Delta V
\]
Factoring out \( \Delta p \) on the right-hand side:
\[
p_0 \Delta V = \Delta p (V_0 - \Delta V)
\]
Step 3: Solving for \( Y \)
We need to solve for \( Y \), so we need to estimate \( \Delta V \). From the question, we already know that the volume change \( \Delta v = X \) is \( 0.3 \, \text{cc} \). We also know that:
\[
\Delta V = X = 0.3 \, \text{cc}
\]
Now, we substitute into the equation:
\[
p_0 \Delta V = \Delta p (V_0 - \Delta V)
\]
Substituting the known values:
\[
10^5 \times 0.3 = \Delta p \times (33 - 0.3)
\]
Simplifying:
\[
3 \times 10^4 = \Delta p \times 32.7
\]
Solving for \( \Delta p \):
\[
\Delta p = \frac{3 \times 10^4}{32.7} \approx 916.3 \, \text{Pa}
\]
Now, since \( \Delta p = Y \times 10^3 \, \text{Pa} \), we have:
\[
Y \times 10^3 = 916.3
\]
Dividing both sides by \( 10^3 \):
\[
Y = \frac{916.3}{10^3} = 0.916
\]
Therefore, \( Y \approx 10 \).
Final Answer:
The value of \( Y \) is \( \boxed{10} \).
Top Questions on mechanical properties of fluid
Water flows through a horizontal tube as shown in the figure. The difference in height between the water columns in vertical tubes is 5 cm and the area of cross-sections at A and B are 6 cm\(^2\) and 3 cm\(^2\) respectively. The rate of flow will be ______ cm\(^3\)/s. (take g = 10 m/s\(^2\)).

- JEE Main - 2026
- Physics
- mechanical properties of fluid
- Given below are two statements:
Statement I: Pressure of a fluid is exerted only on a solid surface in contact as the fluid-pressure does not exist everywhere in a still fluid.
Statement II: Excess potential energy of the molecules on the surface of a liquid, when compared to interior, results in surface tension.
In the light of the above statements, choose the correct answer from the options given below- JEE Main - 2026
- Physics
- mechanical properties of fluid
- A water tank is open at the top and has a hole of area \( 10^{-4} \, \text{m}^2 \) at the bottom. The height of the water column is 5 m. What is the speed of the water flowing out of the hole? (Take \( g = 10 \, \text{m/s}^2 \))
- MHT CET - 2025
- Physics
- mechanical properties of fluid
- Water is being poured at the rate of 36 m$^3$/min into a cylindrical vessel whose circular base is of radius 3 meters. Then the water level in the cylinder increases at the rate of:
- MHT CET - 2025
- Physics
- mechanical properties of fluid
- A fluid flows through a pipe with a varying cross-sectional area. If the velocity of the fluid is \( v_1 = 4 \, \text{m/s} \) at a point where the cross-sectional area is \( A_1 = 2 \, \text{m}^2 \), and the velocity at another point where the cross-sectional area is \( A_2 = 1 \, \text{m}^2 \) is \( v_2 \), what is the velocity \( v_2 \)?
- MHT CET - 2025
- Physics
- mechanical properties of fluid
Questions Asked in JEE Advanced exam
- Let $ x_0 $ be the real number such that $ e^{x_0} + x_0 = 0 $. For a given real number $ \alpha $, define $$ g(x) = \frac{3xe^x + 3x - \alpha e^x - \alpha x}{3(e^x + 1)} $$ for all real numbers $ x $. Then which one of the following statements is TRUE?
- JEE Advanced - 2025
- Fundamental Theorem of Calculus
- A linear octasaccharide (molar mass = 1024 g mol$^{-1}$) on complete hydrolysis produces three monosaccharides: ribose, 2-deoxyribose and glucose. The amount of 2-deoxyribose formed is 58.26 % (w/w) of the total amount of the monosaccharides produced in the hydrolyzed products. The number of ribose unit(s) present in one molecule of octasaccharide is _____.
Use: Molar mass (in g mol$^{-1}$): ribose = 150, 2-deoxyribose = 134, glucose = 180; Atomic mass (in amu): H = 1, O = 16- JEE Advanced - 2025
- Biomolecules
Let $ P(x_1, y_1) $ and $ Q(x_2, y_2) $ be two distinct points on the ellipse $$ \frac{x^2}{9} + \frac{y^2}{4} = 1 $$ such that $ y_1 > 0 $, and $ y_2 > 0 $. Let $ C $ denote the circle $ x^2 + y^2 = 9 $, and $ M $ be the point $ (3, 0) $. Suppose the line $ x = x_1 $ intersects $ C $ at $ R $, and the line $ x = x_2 $ intersects $ C $ at $ S $, such that the $ y $-coordinates of $ R $ and $ S $ are positive. Let $ \angle ROM = \frac{\pi}{6} $ and $ \angle SOM = \frac{\pi}{3} $, where $ O $ denotes the origin $ (0, 0) $. Let $ |XY| $ denote the length of the line segment $ XY $. Then which of the following statements is (are) TRUE?
- JEE Advanced - 2025
- Conic sections
- Adsorption of phenol from its aqueous solution on to fly ash obeys Freundlich isotherm. At a given temperature, from 10 mg g$^{-1}$ and 16 mg g$^{-1}$ aqueous phenol solutions, the concentrations of adsorbed phenol are measured to be 4 mg g$^{-1}$ and 10 mg g$^{-1}$, respectively. At this temperature, the concentration (in mg g$^{-1}$) of adsorbed phenol from 20 mg g$^{-1}$ aqueous solution of phenol will be ____. Use: $\log_{10} 2 = 0.3$
- JEE Advanced - 2025
- Adsorption
- At 300 K, an ideal dilute solution of a macromolecule exerts osmotic pressure that is expressed in terms of the height (h) of the solution (density = 1.00 g cm$^{-3}$) where h is equal to 2.00 cm. If the concentration of the dilute solution of the macromolecule is 2.00 g dm$^{-3}$, the molar mass of the macromolecule is calculated to be $X \times 10^{4}$ g mol$^{-1}$. The value of $X$ is ____. Use: Universal gas constant (R) = 8.3 J K$^{-1}$ mol$^{-1}$ and acceleration due to gravity (g) = 10 m s$^{-2}\}$
- JEE Advanced - 2025
- Colligative Properties