A metal block of base area \(0.20 \,m^2\) is placed on a table, as shown in figure. A liquid film of thickness \(0.25\, mm\) is inserted between the block and the table. The block is pushed by a horizontal force of 0.1 N and moves with a constant speed. If the viscosity of the liquid is \(5.0×10^{−3}Pl\), the speed of block is ________ \(×10^{−3}m/s\)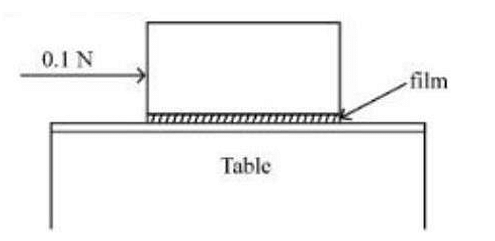

Correct Answer: 25
Solution and Explanation
Top Questions on Viscosity
- For an application where the Reynolds number is to be kept constant, a liquid with a density of 1 g cm\(^-3\) and viscosity of 0.01 Poise results in a characteristic speed of 1 cm s\(^-1\). If this liquid is replaced by another with a density of 1.25 g cm\(^-3\) and viscosity of 0.015 Poise, the characteristic velocity will be ......... cm s\(^-1\) (rounded off to one decimal place).
In an experiment to determine the figure of merit of a galvanometer by half deflection method, a student constructed the following circuit. He applied a resistance of \( 520 \, \Omega \) in \( R \). When \( K_1 \) is closed and \( K_2 \) is open, the deflection observed in the galvanometer is 20 div. When \( K_1 \) is also closed and a resistance of \( 90 \, \Omega \) is removed in \( S \), the deflection becomes 13 div. The resistance of galvanometer is nearly:

- KCET - 2025
- Physics
- Viscosity
- Calculate the Reynolds number for a liquid with density 1 g/cm³ and viscosity 8 × 10⁻⁴ Pa·s, flowing at 0.5 m/s through a pipe of diameter 4 cm.
- SRMJEEE - 2025
- Physics
- Viscosity
- The diameter of spherical galena particles that have the same settling velocity as spherical quartz particles of diameter 25 μm (both settling in water) is ............. μm (rounded off to one decimal place). Assume Stokes law of settling to be valid. Given: Density of galena = 7400 kg m$^{-3}$, density of quartz = 2600 kg m$^{-3}$, density of water = 1000 kg m$^{-3}$.
- For an application where the Reynolds number is to be kept constant, a liquid with density of 1 g per cubic cm and viscosity of 0.01 Poise results in a characteristic speed of 1 cm per second. If this liquid is replaced by another with density of 1.25 g per cubic cm and viscosity of 0.015 Poise, the characteristic velocity will be ........... cm per second (rounded off to one decimal place). Assume the characteristic length of the flow to be the same in both cases.
Questions Asked in JEE Main exam
Which one of the following graphs accurately represents the plot of partial pressure of CS₂ vs its mole fraction in a mixture of acetone and CS₂ at constant temperature?


- JEE Main - 2026
- Organic Chemistry
- Let \( ABC \) be an equilateral triangle with orthocenter at the origin and the side \( BC \) lying on the line \( x+2\sqrt{2}\,y=4 \). If the coordinates of the vertex \( A \) are \( (\alpha,\beta) \), then the greatest integer less than or equal to \( |\alpha+\sqrt{2}\beta| \) is:
- JEE Main - 2026
- Coordinate Geometry
- Three charges $+2q$, $+3q$ and $-4q$ are situated at $(0,-3a)$, $(2a,0)$ and $(-2a,0)$ respectively in the $x$-$y$ plane. The resultant dipole moment about origin is ___.
- JEE Main - 2026
- Electromagnetic waves
Let \( \alpha = \dfrac{-1 + i\sqrt{3}}{2} \) and \( \beta = \dfrac{-1 - i\sqrt{3}}{2} \), where \( i = \sqrt{-1} \). If
\[ (7 - 7\alpha + 9\beta)^{20} + (9 + 7\alpha - 7\beta)^{20} + (-7 + 9\alpha + 7\beta)^{20} + (14 + 7\alpha + 7\beta)^{20} = m^{10}, \] then the value of \( m \) is ___________.- JEE Main - 2026
- Complex Numbers and Quadratic Equations
- The work functions of two metals ($M_A$ and $M_B$) are in the 1 : 2 ratio. When these metals are exposed to photons of energy 6 eV, the kinetic energy of liberated electrons of $M_A$ : $M_B$ is in the ratio of 2.642 : 1. The work functions (in eV) of $M_A$ and $M_B$ are respectively.
- JEE Main - 2026
- Dual nature of matter