X and Y in the following reactions, respectively, are
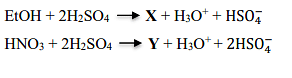
- CH3COOH and NO+
- CH3CHO and NO2\(^+_2\)
- EtOSO3H and NO2\(^+_2\)
- EtOSO3H and NO+
The Correct Option is C
Solution and Explanation
To determine the compounds X and Y in the given reactions, we need to analyze them based on known chemical reactions involving sulfuric acid (H₂SO₄).
Reaction 1: EtOH (Ethanol) + 2 H₂SO₄
When ethanol reacts with concentrated sulfuric acid, it forms ethyl hydrogen sulfate (EtOSO₃H) and water. The reaction is:
\[\text{EtOH} + \text{H}_2\text{SO}_4 \rightarrow \text{EtOSO}_3\text{H} + \text{H}_3\text{O}^+ + \text{HSO}_4^-\]
This indicates that compound X is ethyl hydrogen sulfate (EtOSO₃H).
Reaction 2: HNO₃ + 2 H₂SO₄
When nitric acid (HNO₃) reacts with sulfuric acid, it forms the nitronium ion (NO₂⁺) which is used as an electrophile in nitration reactions. The relevant reaction is:
\[\text{HNO}_3 + 2\text{H}_2\text{SO}_4 \rightarrow \text{NO}_2^+ + \text{H}_3\text{O}^+ + 2\text{HSO}_4^-\]
Thus, compound Y is the nitronium ion (NO₂⁺).
Conclusion: Therefore, the correct answer is that X is EtOSO₃H (ethyl hydrogen sulfate) and Y is NO₂⁺ (nitronium ion).
Top Questions on Heterocyclic Chemistry
- The acidity of the compounds shown below
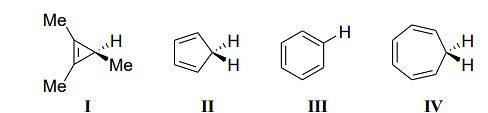
follows the order- IIT JAM CY - 2024
- Organic Chemistry
- Heterocyclic Chemistry
- Famotidine contains:
- GPAT - 2024
- Organic Chemistry
- Heterocyclic Chemistry
- The IUPAC name of tartaric acid is:
- GPAT - 2024
- Organic Chemistry
- Heterocyclic Chemistry
- The IUPAC name of tartaric acid is:
- GPAT - 2024
- Organic Chemistry
- Heterocyclic Chemistry
- Famotidine contains:
- GPAT - 2024
- Organic Chemistry
- Heterocyclic Chemistry
Questions Asked in IIT JAM CY exam
- Among the following, the correct condition(s) for spontaneity is(are)
- IIT JAM CY - 2025
- Thermodynamics
One mole of a monoatomic ideal gas starting from state A, goes through B and C to state D, as shown in the figure. Total change in entropy (in J K\(^{-1}\)) during this process is ...............

- IIT JAM CY - 2025
- Thermodynamics
The number of chiral carbon centers in the following molecule is ...............

- IIT JAM CY - 2025
- General Chemistry
- Consider the following matrices A and B.
\[ A = \begin{pmatrix} 1 & 2 & 0 & 0 \\ 3 & 4 & 0 & 0 \\ 0 & 5 & 5 & 0 \\ 0 & 0 & 6 & 7 \\ 0 & 0 & 8 & 9 \end{pmatrix} \quad \text{and} \quad B = \begin{pmatrix} 10 & 11 & 0 & 0 & 0 \\ 12 & 13 & 0 & 0 & 0 \\ 0 & 0 & 4 & 0 & 0 \\ 0 & 0 & 15 & 16 & 0 \\ 0 & 0 & 17 & 18 & 0 \end{pmatrix} \]
If \( C = AB \), the sum of the diagonal elements of \( C \) is ..............
- IIT JAM CY - 2025
- General Chemistry
A tube fitted with a semipermeable membrane is dipped into 0.001 M NaCl solution at 300 K as shown in the figure. Assume density of the solvent and solution are the same. At equilibrium, the height of the liquid column \( h \) (in cm) is .........

- IIT JAM CY - 2025
- General Chemistry