Which of the following does not form a buffer solution?
Which of the following does not form a buffer solution?
NH3+HCL (2:1 mole ratio)
CH3CO2H + NaOH (2:1 mole ratio)
$NaOH + CH_3COOH$ (1:1 mole ratio)
NH4Cl + NH3 (1:1 mole ratio)
The Correct Option is C
Solution and Explanation
To solve the problem, we need to identify which combination does not form a buffer solution.
1. Understanding Buffer Solutions:
A buffer solution resists changes in pH when small amounts of acid or base are added. Buffers are typically formed by:
- A weak acid and its salt with a strong base (e.g., CH₃COOH + CH₃COONa)
- A weak base and its salt with a strong acid (e.g., NH₃ + NH₄Cl)
The key is having a weak acid/base and its conjugate pair in proper proportions.
2. Analyze Each Option:
- Option 1: NH₃ + HCl (2:1 mole ratio)
NH₃ is a weak base and HCl is a strong acid. At a 2:1 ratio, not all NH₃ reacts with HCl, so some NH₃ and NH₄⁺ (from NH₄Cl) remain — this forms a buffer. - Option 2: CH₃COOH + NaOH (2:1 mole ratio)
Excess CH₃COOH remains after partial neutralization by NaOH, forming CH₃COONa and CH₃COOH — a buffer system. - Option 3: NaOH + CH₃COOH (1:1 mole ratio)
NaOH completely neutralizes CH₃COOH to form only CH₃COONa. No weak acid (CH₃COOH) remains, so this is not a buffer. - Option 4: NH₄Cl + NH₃ (1:1 mole ratio)
This is a classic weak base (NH₃) and its salt (NH₄Cl) — it forms a buffer.
Final Answer:
The correct answer is NaOH + CH₃COOH (1:1 mole ratio) because it does not form a buffer solution.
Top Questions on Law Of Chemical Equilibrium And Equilibrium Constant
- Consider the following gaseous equilibrium in a closed container of volume $V$ at temperature $T$:
P$_2$(g) + Q$_2$(g) $\rightleftharpoons$ 2PQ(g)
Initially, 2 moles each of P$_2$(g), Q$_2$(g) and PQ(g) are present at equilibrium. One mole each of P$_2$ and Q$_2$ are added. The number of moles of P$_2$, Q$_2$ and PQ at the new equilibrium respectively are
- JEE Main - 2026
- Chemistry
- Law Of Chemical Equilibrium And Equilibrium Constant
- $X_2(g) + Y_2(g) \rightleftharpoons 2Z(g)$. Equilibrium moles of $X_2, Y_2, Z$ are 3, 3, 9 mol (in 1 L). 10 mol of Z(g) is added. New equilibrium moles of Z(g) is ___. (Nearest integer)
- JEE Main - 2026
- Chemistry
- Law Of Chemical Equilibrium And Equilibrium Constant
- Observe the following equilibrium in a 1 L flask:
A(g) <=> B(g)
At temperature T (in K), the equilibrium concentrations of A and B are 0.5 M and 0.375 M respectively.
Then 0.1 moles of A is added into the flask and the system is heated to temperature T again to re-establish equilibrium.
The new equilibrium concentrations (in M) of A and B respectively are:- JEE Main - 2026
- Chemistry
- Law Of Chemical Equilibrium And Equilibrium Constant
- The plot of \(\log_{10}K\) vs \(\frac{1}{T}\) gives a straight line. The intercept and slope respectively are (where \(K\) is equilibrium constant).
- JEE Main - 2026
- Chemistry
- Law Of Chemical Equilibrium And Equilibrium Constant
- For the reaction \( \mathrm{A \rightleftharpoons B} \), the number of moles of A and B at equilibrium in a \(1\,\text{L}\) vessel are \(0.50\) and \(0.375\), respectively. If \(0.10\) mol of A is added further, determine the number of moles of A and B at the new equilibrium.
- JEE Main - 2026
- Chemistry
- Law Of Chemical Equilibrium And Equilibrium Constant
Questions Asked in TS EAMCET exam
- A body is projected vertically upward with an initial velocity of 40 m/s. Calculate the maximum height reached by the body. (Take \( g = 9.8 \, \text{m/s}^2 \))
- TS EAMCET - 2025
- Kinematics
- The properties required for a material to be used as the core of an electromagnet are:
- TS EAMCET - 2025
- Magnetism and matter
- Which of the following statements are correct?
- TS EAMCET - 2025
- Colligative Properties
- At 27$^\circ$C, 100 mL of 0.05 M Cu$^{2+}$ solution is added to 1 L of 0.1 M KI. Find [KI] in resultant solution.
- TS EAMCET - 2025
- Stoichiometry and Stoichiometric Calculations
- 4.0 g of a mixture containing Na₂CO₃ and NaHCO₃ is heated to 673K. Loss in mass of the mixture is found to be 0.62g. The percentage of sodium carbonate in the mixture is
- TS EAMCET - 2025
- Stoichiometry and Stoichiometric Calculations
Concepts Used:
Law of Chemical Equilibrium
Law of Chemical Equilibrium states that at a constant temperature, the rate of a chemical reaction is directly proportional to the product of the molar concentrations of the reactants each raised to a power equal to the corresponding stoichiometric coefficients as represented by the balanced chemical equation.
Let us consider a general reversible reaction;
A+B ↔ C+D
After some time, there is a reduction in reactants A and B and an accumulation of the products C and D. As a result, the rate of the forward reaction decreases and that of backward reaction increases.
Eventually, the two reactions occur at the same rate and a state of equilibrium is attained.
By applying the Law of Mass Action;
The rate of forward reaction;
Rf = Kf [A]a [B]b
The rate of backward reaction;
Rb = Kb [C]c [D]d
Where,
[A], [B], [C] and [D] are the concentrations of A, B, C and D at equilibrium respectively.
a, b, c, and d are the stoichiometric coefficients of A, B, C and D respectively.
Kf and Kb are the rate constants of forward and backward reactions.
However, at equilibrium,
Rate of forward reaction = Rate of backward reaction.
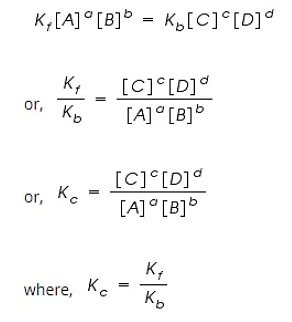
Kc is called the equilibrium constant expressed in terms of molar concentrations.
The above equation is known as the equation of Law of Chemical Equilibrium.