Question:
Which of the following compound does not exhibit geometrical Isomerism?
Which of the following compound does not exhibit geometrical Isomerism?
Show Hint
When looking for geometrical isomerism, check for compounds that have double bonds or cyclic structures that restrict rotation.
Updated On: May 13, 2025
- 2- Butene
- 3- Hexene
- But- 2- enal
- Styrene
Hide Solution
Verified By Collegedunia
The Correct Option is D
Solution and Explanation
Geometrical isomerism is exhibited by compounds with restricted rotation around a double bond. Styrene, being a simple alkene, does not exhibit such isomerism.
Was this answer helpful?
0
0
Top Questions on Optical and geometrical isomerism
- Upon addition of compound ( X ) to an aqueous AgNO(_3) solution, a white precipitate appears instantly. Also, ( X ) does not exhibit geometrical isomerism. The CORRECT option(s) for ( X ) is/are:
- GATE XL - 2024
- Chemistry
- Optical and geometrical isomerism
- The molecules A and B are a pair of___
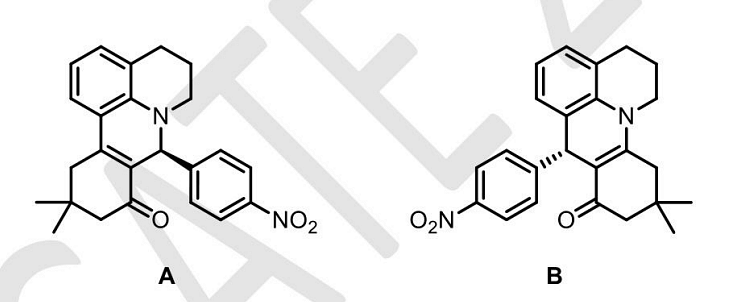
- GATE XL - 2024
- Chemistry
- Optical and geometrical isomerism
- The molecules A and B are a pair of___

- GATE XL - 2024
- Chemistry
- Optical and geometrical isomerism
- Phenol is manufactured from hydrocarbon, Cumene. Cumene is chemically:
- CUET (UG) - 2023
- Chemistry
- Optical and geometrical isomerism
Questions Asked in AP EAPCET exam
- In a series LCR circuit, the voltages across the capacitor, resistor, and inductor are in the ratio 2:3:6. If the voltage of the source in the circuit is 240 V, then the voltage across the inductor is
- AP EAPCET - 2025
- Electromagnetic induction
- 0.25 moles of $ \text{CH}_2\text{FCOOH} $ was dissolved in $ 0.5 \, \text{kg} $ of water. The depression in freezing point of the resultant solution was observed as $ 1^\circ \text{C} $. What is the van't Hoff factor? ($ K_f = 1.86 \, \text{K kg mol}^{-1} $)
- AP EAPCET - 2025
- Colligative Properties
- At $T(K)$, the vapor pressure of water is $x$ kPa. What is the vapor pressure (in kPa) of 1 molal solution containing non-volatile solute?
- AP EAPCET - 2025
- Colligative Properties
- At 300 K, vapour pressure of pure liquid A is 70 mm Hg. It forms an ideal solution with liquid B. Mole fraction of B = 0.2 and total vapour pressure of solution = 84 mm Hg. What is vapour pressure (in mm) of pure B?
- AP EAPCET - 2025
- Colligative Properties
- A 1% (w/v) aqueous solution of a certain solute is isotonic with a 3% (w/v) solution of glucose (molar mass 180 g mol$^{-1}$). The molar mass of solute (in g mol$^{-1}$) is
- AP EAPCET - 2025
- Colligative Properties
View More Questions