The major product formed in the above acid‐catalyzed reaction is:
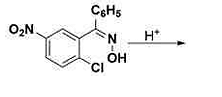

The Correct Option is D
Solution and Explanation
The given reaction is an acid-catalyzed transformation involving a nitro-substituted hydroxylamine derivative. The major product is determined by the mechanism of the reaction and the stability of intermediates.
Mechanism Explanation:
- The reaction begins with the protonation of the hydroxylamine group in acidic medium, making it a better leaving group.
- The loss of water from the protonated intermediate forms a nitrenium ion.
- This nitrenium ion is highly reactive and undergoes a rearrangement or further reaction to form a stable aromatic compound.
- Considering the substituents on the aromatic ring, 1,2 or 1,4 rearrangements are feasible.
- The presence of electron-withdrawing groups like the nitro group facilitates the rearrangement leading to the most stable product.
Based on the rearrangements possible and the stabilizing effect of substituents, the major product is predicted to be the one where the aromatic ring regains its aromaticity and stability.
Conclusion: The presence of the nitro and chloro groups along with the reaction conditions favors the formation of this particular product, making it the most stable configuration post-reaction.
Top Questions on Polycyclic aromatic hydrocarbons
- Which of the following compounds will have the highest order of their reactivity towards electrophilic substitution reaction?

Choose the correct answer from the options given below:- CUET (PG) - 2024
- Chemistry
- Polycyclic aromatic hydrocarbons
- Which of the following molecules is aromatic?

- CUET (PG) - 2024
- Chemistry
- Polycyclic aromatic hydrocarbons
- Identify the product in the following chemical reaction:
- CUET (PG) - 2024
- Chemistry
- Polycyclic aromatic hydrocarbons
- Which of the following isomer of 1, 2-cyclohexanedicarboxylic acid forms an hydride on heating?

Choose the correct answer from the options given below:- CUET (PG) - 2024
- Chemistry
- Polycyclic aromatic hydrocarbons
- The major product formed in the above reaction is:
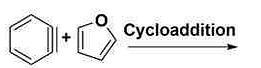
- GPAT - 2022
- Organic Chemistry
- Polycyclic aromatic hydrocarbons
Questions Asked in GPAT exam
- Match the following:
(1) Schedule FF
(2) Schedule F3
(3) Schedule V
(4) Schedule Y
Descriptions:
(P) Standards of patent and proprietary medicines
(Q) Requirements and guidelines for clinical trials
(R) Standards for sterilized umbilical tapes
(S) Standards for Ophthalmic preparations- GPAT - 2025
- Drug therapy
- If the label or the container bears the name of an individual or company purporting to be the manufacturer of the drug, which individual or company is fictitious or does not exist, it is:
- GPAT - 2025
- Drug therapy
- Manufacturing Specification for tooling has been standardized by?
- GPAT - 2025
- Pharmacy Profession & Introduction to Pharmaceuticals
- As per USP, the maximum concentration of benzalkonium chloride used as a preservative in parenteral formulations is
- GPAT - 2025
- Pharmaceutical Analysis
Match the following:
(P) Schedule H
(Q) Schedule G
(R) Schedule P
(S) Schedule F2
Descriptions:
(I) Life period of drugs
(II) Drugs used under RMP
(III) List of Prescription Drugs
(IV) Standards for surgical dressing
- GPAT - 2025
- Drug therapy



