Replacement of the diazonium group by halogen in presence of copper powder is:
- Gabriel reaction
- Gattermann reaction
- Hofmann reaction
- Sandmeyer reaction
The Correct Option is D
Solution and Explanation
To solve the question about the replacement of the diazonium group by halogen in the presence of copper powder, we need to understand the reactions in the context of organic chemistry.
Let's look at the options provided:
- Gabriel Reaction: This is primarily used for the synthesis of primary amines and does not involve diazonium groups or halogens.
- Gattermann Reaction: This involves the introduction of a formyl group (CHO) into aromatic compounds and uses a mixture of HCN and HCl in the presence of a Lewis acid, such as AlCl3. It does not specifically involve diazonium groups being replaced by halogens.
- Hofmann Reaction: This is used to convert amides to amines, with the loss of one carbon atom. It does not involve copper powder or diazonium groups.
- Sandmeyer Reaction: This is a reaction where an aryl diazonium compound is transformed into an aryl halide using copper(I) chloride or copper(I) bromide. The reaction indeed involves the replacement of the diazonium group with a halogen in the presence of copper (or copper salts), making it the correct answer.
Conclusion: The correct answer is the Sandmeyer reaction. In this reaction, copper powder or copper salts are used to facilitate the replacement of the diazonium group by a halogen.
This reaction is valuable in the preparation of aryl halides from anilines, where initially, the amine group is converted into a diazonium salt, and then further treated with halide ions in the presence of copper to produce the desired aryl halide.
Top Questions on Mass spectrometry
- The major product in the given reaction sequence is Q. The mass spectrum of Q shows
([M] = molecular ion peak)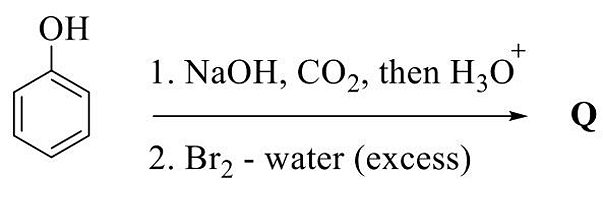
- GATE CY - 2024
- Inorganic Chemistry
- Mass spectrometry
- Recombination of electron-hole produces ____ in LEDs.
- AP PGECET - 2024
- Instrumentation Engineering
- Mass spectrometry
- Which among the following characteristics of Laser light specifies the precise movement of all individual light waves together through time and space?
- AP PGECET - 2024
- Instrumentation Engineering
- Mass spectrometry
- Magnetic sector analyzer is a part of
- AP PGECET - 2024
- Instrumentation Engineering
- Mass spectrometry
- Which ionization technique in mass spectrometry is most suitable for large biomolecules like proteins:
- GPAT - 2024
- Pharmaceutical Analysis
- Mass spectrometry
Questions Asked in GPAT exam
- Match the following:
(1) Schedule FF
(2) Schedule F3
(3) Schedule V
(4) Schedule Y
Descriptions:
(P) Standards of patent and proprietary medicines
(Q) Requirements and guidelines for clinical trials
(R) Standards for sterilized umbilical tapes
(S) Standards for Ophthalmic preparations- GPAT - 2025
- Drug therapy
- If the label or the container bears the name of an individual or company purporting to be the manufacturer of the drug, which individual or company is fictitious or does not exist, it is:
- GPAT - 2025
- Drug therapy
- Manufacturing Specification for tooling has been standardized by?
- GPAT - 2025
- Pharmacy Profession & Introduction to Pharmaceuticals
- As per USP, the maximum concentration of benzalkonium chloride used as a preservative in parenteral formulations is
- GPAT - 2025
- Pharmaceutical Analysis
Match the following:
(P) Schedule H
(Q) Schedule G
(R) Schedule P
(S) Schedule F2
Descriptions:
(I) Life period of drugs
(II) Drugs used under RMP
(III) List of Prescription Drugs
(IV) Standards for surgical dressing
- GPAT - 2025
- Drug therapy