Orbital is :
- circular path around the nucleus in which the electrons revolves
- space around the nucleus where the probablity of finding the electron is maximum
- amplitude of electron wave
- none of the above
The Correct Option is B
Solution and Explanation
Top Questions on Molecular Orbital Theory
- Pair of species among the following having same bond order as well as paramagnetic character will be:
- JEE Main - 2026
- Chemistry
- Molecular Orbital Theory
- Among the species O$_2^+$, N$_2^-$, N$_2^{2-}$ and O$_2^-$ which have same bond order as well as paramagnetic in nature.
- JEE Main - 2026
- Chemistry
- Molecular Orbital Theory
Regarding the molecular orbital (MO) energy levels for homonuclear diatomic molecules, the INCORRECT statement(s) is (are):
- JEE Advanced - 2025
- Chemistry
- Molecular Orbital Theory
- Arrange the following in increasing order of bond order: (A) He\(_2^+\)
(B) O\(_2^-\)
(C) HF
(D) NO\(^-\)- CUET (PG) - 2025
- Chemistry
- Molecular Orbital Theory
- Which of the following is the ratio of 5\(^\text{th}\) Bohr orbit \( (r_5) \) of He\(^+\) & Li\(^{2+}\)?
- JEE Main - 2025
- Chemistry
- Molecular Orbital Theory
Questions Asked in NEET exam
- A chemical reaction proceeds into the following steps Step I,$\hspace15mm 2A \rightleftharpoons X$ fast Step II,$\hspace10mm X + B \rightleftharpoons Y$ slow Step III,$\hspace10mm Y + B \rightleftharpoons $ Product fast The law for the overall reaction is
- Delhi UMET/DPMT - 2011
- Chemical Kinetics
- A solution is $0.1 M$ with respect to $Ag ^{+}, Ca ^{2+}, Mg ^{2+}$ and $Al ^{3+}$, which will precipitate at lowest concentration of $\left[ PO _{4}^{3-}\right]$ when solution of $Na _{3} PO _{4}$ is added?
- Delhi UMET/DPMT - 2011
- Equilibrium
- How many $ 1\,\mu F $ capacitors must be connected in parallel to store a charge of $1\, C$ with a potential of $110\, V$ across the capacitors?
- Delhi UMET/DPMT - 2011
- electrostatic potential and capacitance
- Most stable carbocation is
- Delhi UMET/DPMT - 2011
- Organic Chemistry
- Given a gas phase reaction, $\hspace10mm 2A(g)+B(g) \rightleftharpoons C(g)+D(g)$ Which one of the following changes will affect the value of $K_c$?
- Delhi UMET/DPMT - 2011
- Equilibrium
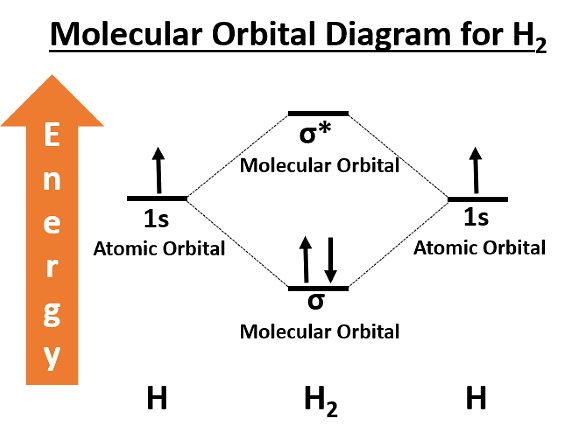
Concepts Used:
Molecular Orbital Theory
The Molecular Orbital Theory is a more sophisticated model of chemical bonding where new molecular orbitals are generated using a mathematical process called Linear Combination of Atomic Orbitals (LCAO).
Molecular Orbital theory is a chemical bonding theory that states that individual atoms combine together to form molecular orbitals. Due to this arrangement in MOT Theory, electrons associated with different nuclei can be found in different atomic orbitals. In molecular orbital theory, the electrons present in a molecule are not assigned to individual chemical bonds between the atoms. Rather, they are treated as moving under the influence of the atomic nuclei in the entire molecule.