Match List I with List II :
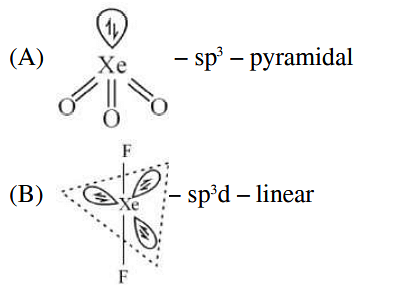
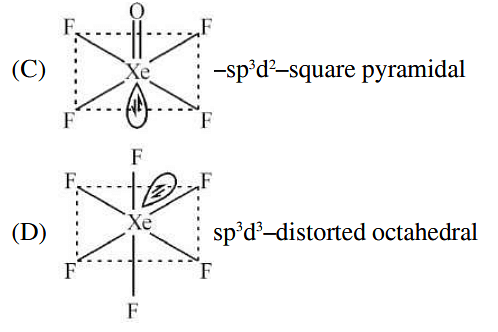
List-I (molecule) List-II (hybridization; shape) A XeO3 i sp3d ; linear B XeF2 ii sp3; pyramidal C XeOF4 iii sp3d3; distorted octahedral D XeF6 iv sp3d2 ;square pyramidal
Choose the correct answer from the options given below:
Match List I with List II :
| List-I (molecule) | List-II (hybridization; shape) | ||
|---|---|---|---|
| A | XeO3 | i | sp3d ; linear |
| B | XeF2 | ii | sp3; pyramidal |
| C | XeOF4 | iii | sp3d3; distorted octahedral |
| D | XeF6 | iv | sp3d2 ;square pyramidal |
Choose the correct answer from the options given below:
- A-II, B-I, C-IV, D-III
- A-II, B-IV, C-III, D-I
- A-IV, B-II, C-III, D-I
- A-IV, B-II, C-I, D-III
The Correct Option is A
Solution and Explanation
Top Questions on Chemical bonding and molecular structure
From the given following (A to D) cyclic structures, those which will not react with Tollen's reagent are :

- JEE Main - 2026
- Chemistry
- Chemical bonding and molecular structure
- The wave numbers of three spectral lines of hydrogen atom are considered. Identify the set of spectral lines belonging to the {Balmer series. (\(R\) = Rydberg constant)}
- JEE Main - 2026
- Chemistry
- Chemical bonding and molecular structure
- Consider the reaction: \[ \text{Ph–CH=CH}_2 \xrightarrow[\text{peroxide}]{\text{HBr}} \text{Product} \] Which of the following statements are correct?
[A.] The reaction proceeds through a more stable radical intermediate.
[B.] The role of peroxide is to generate \(\mathrm{H^\bullet}\) radical.
[C.] During this reaction, benzene is formed as a byproduct.
[D.] \(1\)-Bromo-\(2\)-phenylethane is formed as a minor product.
[E.] The same reaction in absence of peroxide proceeds via a carbocation intermediate. Choose the correct answer.- JEE Main - 2026
- Chemistry
- Chemical bonding and molecular structure
Compound 'P' undergoes the following sequence of reactions : (i) NH₃ (ii) $\Delta$ $\rightarrow$ Q (i) KOH, Br₂ (ii) CHCl₃, KOH (alc), $\Delta$ $\rightarrow$ NC-CH₃. 'P' is :


- JEE Main - 2026
- Chemistry
- Chemical bonding and molecular structure
- Given below are two statements :
Statement I : The correct order in terms of bond dissociation enthalpy is \( Cl_2>Br_2>F_2>I_2 \).
Statement II : The correct trend in the covalent character of the metal halides is \( SnCl_2>SnCl_4 \), \( PbCl_2>PbCl_4 \) and \( UF_4>UF_6 \).
In the light of the above statements, choose the correct answer from the options given below :- JEE Main - 2026
- Chemistry
- Chemical bonding and molecular structure
Questions Asked in JEE Main exam
- In a microscope of tube length $10\,\text{cm}$ two convex lenses are arranged with focal lengths $2\,\text{cm}$ and $5\,\text{cm}$. Total magnification obtained with this system for normal adjustment is $(5)^k$. The value of $k$ is ___.
- JEE Main - 2026
- Optical Instruments
Which one of the following graphs accurately represents the plot of partial pressure of CS₂ vs its mole fraction in a mixture of acetone and CS₂ at constant temperature?


- JEE Main - 2026
- Organic Chemistry
- Let \( ABC \) be an equilateral triangle with orthocenter at the origin and the side \( BC \) lying on the line \( x+2\sqrt{2}\,y=4 \). If the coordinates of the vertex \( A \) are \( (\alpha,\beta) \), then the greatest integer less than or equal to \( |\alpha+\sqrt{2}\beta| \) is:
- JEE Main - 2026
- Coordinate Geometry
- Three charges $+2q$, $+3q$ and $-4q$ are situated at $(0,-3a)$, $(2a,0)$ and $(-2a,0)$ respectively in the $x$-$y$ plane. The resultant dipole moment about origin is ___.
- JEE Main - 2026
- Electromagnetic waves
Let \( \alpha = \dfrac{-1 + i\sqrt{3}}{2} \) and \( \beta = \dfrac{-1 - i\sqrt{3}}{2} \), where \( i = \sqrt{-1} \). If
\[ (7 - 7\alpha + 9\beta)^{20} + (9 + 7\alpha - 7\beta)^{20} + (-7 + 9\alpha + 7\beta)^{20} + (14 + 7\alpha + 7\beta)^{20} = m^{10}, \] then the value of \( m \) is ___________.- JEE Main - 2026
- Complex Numbers and Quadratic Equations
Concepts Used:
Chemical Bonding and Molecular Structure
Such a group of atoms is called a molecule. Obviously, there must be some force that holds these constituent atoms together in the molecules. The attractive force which holds various constituents (atoms, ions, etc.) together in different chemical species is called a chemical bond.
Types of Chemical Bonds:
There are 4 types of chemical bonds which are formed by atoms or molecules to yield compounds.
- Ionic Bonds - Ionic bonding is a type of chemical bonding which involves a transfer of electrons from one atom or molecule to another.
- Covalent Bonds - Compounds that contain carbon commonly exhibit this type of chemical bonding.
- Hydrogen Bonds - It is a type of polar covalent bonding between oxygen and hydrogen wherein the hydrogen develops a partial positive charge
- Polar Bonds - In Polar Covalent chemical bonding, electrons are shared unequally since the more electronegative atom pulls the electron pair closer to itself and away from the less electronegative atom.
Factors Affecting Bond Enthalpy in Chemical Bonding:
- Size of the Atom
- Multiplicity of Bonds
- Number of Lone Pair of Electrons Present
- Bond Angle