In the reaction :
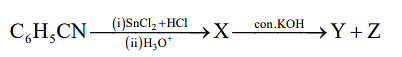
Formation of X, formation of Y and Z are known by
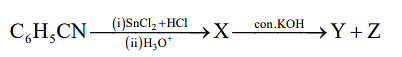
Formation of X, formation of Y and Z are known by
- Rosenmund reduction, Cannizaro reaction
- Clemmensen reduction, Sandmeyer reaction.
- Wolff-Kishner reduction, Wurtz reaction.
- Stephen reaction, Cannizaro reaction
The Correct Option is D
Approach Solution - 1
The given reaction involves the transformation of C6H5CN (benzene nitrile) through several steps. Let's break down each step to understand the formation of X, Y, and Z:
Step 1: C6H5CN (i) SnCl2 + HCl
This is a typical Stephen reaction, where benzene nitrile (C6H5CN) is reduced by tin chloride (SnCl2) in the presence of hydrochloric acid (HCl). This reaction reduces the nitrile group to an imide group, forming benzylamine (C6H5CH2NH2) as X.
Step 2: (ii) H3O+ and con. KOH
This step involves the treatment of the imide with concentrated KOH and H3O+. The Cannizzaro reaction is a disproportionation reaction of aldehydes (or compounds with an aldehyde group), where one molecule is reduced to alcohol and the other oxidized to an acid. Here, benzylamine undergoes this reaction, leading to the formation of Y (benzaldehyde) and Z (formic acid).
Conclusion:
The reactions correspond to the following:
- Formation of X: Stephen reaction (reduction of nitrile to imide)
- Formation of Y and Z: Cannizzaro reaction (disproportionation of aldehyde)
Correct Answer: Option B: Stephen reaction, Cannizzaro reaction
Approach Solution -2
Reaction:
C\(_6\)H\(_5\)CN \(\xrightarrow{\text{SnCl}_2 + \text{HCl}}\) X \(\xrightarrow{\text{con. KOH}}\) Y + Z \(\xrightarrow{\text{H}_2\text{O}}\)
The formation of X, Y, and Z suggests the following reactions:
Step 1: The reaction of C\(_6\)H\(_5\)CN (Benzonitrile) with SnCl\(_2\) in the presence of HCl leads to the reduction of the nitrile group to an aldehyde group, forming X. This is known as the Stephen reaction.
Step 2: When X is treated with conc. KOH, it undergoes an aldol condensation, forming Y and Z. Y is likely to be a product of condensation, and Z could be the byproduct of the reaction. This is characteristic of the Cannizzaro reaction, which involves the disproportionation of aldehydes without an alpha-hydrogen.
Hence, the reactions correspond to the Stephen reaction followed by the Cannizzaro reaction.
Correct Answer: (D) Stephen reaction, Cannizzaro reaction.
Top Questions on introduction to organic chemistry
- Which of the following represents the correct IUPAC name for \( \text{CH}_3\text{CH}_2\text{CH}_2\text{OH} \)?
- MHT CET - 2025
- Chemistry
- introduction to organic chemistry
- Which of the following represents the correct IUPAC name for \( \text{CH}_3\text{CH}_2\text{OH} \)?
- MHT CET - 2025
- Chemistry
- introduction to organic chemistry
- What is the IUPAC name of CH$_3$CH(OH)CH$_3$?
- BITSAT - 2025
- Chemistry
- introduction to organic chemistry
- What is the concentration of \( \text{H}^+ \) ions if the pH is 2.7?
- MHT CET - 2024
- Chemistry
- introduction to organic chemistry
- Calculate the pH of the solution using the Henderson-Hasselbalch equation.
- MHT CET - 2024
- Chemistry
- introduction to organic chemistry
Questions Asked in KCET exam
Match the following:
In the following, \( [x] \) denotes the greatest integer less than or equal to \( x \).
Choose the correct answer from the options given below:- KCET - 2025
- Differentiability
- If \[ y = \frac{\cos x}{1 + \sin x} \] then:
- KCET - 2025
- Differentiability
- A function \( f(x) \) is given by:
\[ f(x) = \begin{cases} \frac{1}{e^x - 1}, & \text{if } x \neq 0 \\ \frac{1}{e^x + 1}, & \text{if } x = 0 \end{cases} \] Then, which of the following is true?- KCET - 2025
- Limits
- The function f(x) is given by:
For x < 0:
f(x) = ex + axFor x ≥ 0:
f(x) = b(x - 1)2
The function is differentiable at x = 0. Then,- KCET - 2025
- Differentiability
- The function \( f(x) = \tan x - x \)
- KCET - 2025
- Derivatives