Identify the correct increasing order of energies of molecular orbitals for F2 molecule.
Identify the correct increasing order of energies of molecular orbitals for F2 molecule.
\(σ_{1s}< σ^{*}_{1s} < σ_{2s} < σ^{*}_{2s}\)
\(σ_{1s}< σ_{2s} <σ^{*}_{1s} < σ^{*}_{2s}\)
\(σ_{1s} < σ^{*}_{1s} < σ^{*}_{2s} < σ_{2s}\)
\(σ ^{*}_{1s} < σ_{1s} < σ^{ *}_{2s} < σ_{2s}\)
The Correct Option is D
Solution and Explanation
Let's break down the options to understand why this is the correct order:
- In the F2 molecule, the \(σ_{1s}\) bonding orbital is formed by the overlap of the two 1s atomic orbitals from each fluorine atom. This bonding orbital is lower in energy compared to the other molecular orbitals.
- The \(σ^{*}_{1s}\) antibonding orbital is formed by the destructive overlap of the two 1s atomic orbitals. It is higher in energy than the \(σ_{1s}\) bonding orbital.
- The \(σ_{2s}\) bonding orbital is formed by the overlap of the two 2s atomic orbitals from each fluorine atom. It is lower in energy than the σ* 1s orbital.
- Finally, the \(σ^{*}_{2s}\) antibonding orbital is formed by the destructive overlap of the two 2s atomic orbitals. It is higher in energy than the \(σ_{2s}\) bonding orbital.
Therefore, the correct order is \(σ^{*}_{1s} < σ_{1s} < σ^{*}_{2s} < σ_{2s}\), which corresponds to option (D).
Top Questions on Chemical bonding and molecular structure
From the given following (A to D) cyclic structures, those which will not react with Tollen's reagent are :

- JEE Main - 2026
- Chemistry
- Chemical bonding and molecular structure
- The wave numbers of three spectral lines of hydrogen atom are considered. Identify the set of spectral lines belonging to the {Balmer series. (\(R\) = Rydberg constant)}
- JEE Main - 2026
- Chemistry
- Chemical bonding and molecular structure
- Consider the reaction: \[ \text{Ph–CH=CH}_2 \xrightarrow[\text{peroxide}]{\text{HBr}} \text{Product} \] Which of the following statements are correct?
[A.] The reaction proceeds through a more stable radical intermediate.
[B.] The role of peroxide is to generate \(\mathrm{H^\bullet}\) radical.
[C.] During this reaction, benzene is formed as a byproduct.
[D.] \(1\)-Bromo-\(2\)-phenylethane is formed as a minor product.
[E.] The same reaction in absence of peroxide proceeds via a carbocation intermediate. Choose the correct answer.- JEE Main - 2026
- Chemistry
- Chemical bonding and molecular structure
Compound 'P' undergoes the following sequence of reactions : (i) NH₃ (ii) $\Delta$ $\rightarrow$ Q (i) KOH, Br₂ (ii) CHCl₃, KOH (alc), $\Delta$ $\rightarrow$ NC-CH₃. 'P' is :


- JEE Main - 2026
- Chemistry
- Chemical bonding and molecular structure
- Given below are two statements :
Statement I : The correct order in terms of bond dissociation enthalpy is \( Cl_2>Br_2>F_2>I_2 \).
Statement II : The correct trend in the covalent character of the metal halides is \( SnCl_2>SnCl_4 \), \( PbCl_2>PbCl_4 \) and \( UF_4>UF_6 \).
In the light of the above statements, choose the correct answer from the options given below :- JEE Main - 2026
- Chemistry
- Chemical bonding and molecular structure
Questions Asked in MHT CET exam
Which part of root absorb mineral?
- MHT CET - 2025
- The Root
- A body of mass 2 kg is moving in a circular path of radius 3 m with a constant speed of 6 m/s. What is the centripetal force acting on the body?
- MHT CET - 2025
- Centripetal forces
- A 200 g sample of water at 80°C is mixed with 100 g of water at 20°C. Assuming no heat loss to the surroundings, what is the final temperature of the mixture?
- MHT CET - 2025
- thermal properties of matter
- Given the equation: \[ 81 \sin^2 x + 81 \cos^2 x = 30 \] Find the value of \( x \).
- MHT CET - 2025
- Trigonometric Identities
- A body of mass 10 kg is at a height of 5 m above the surface of the Earth. What is the gravitational potential energy of the body? (Take \( g = 10 \, \text{m/s}^2 \))
- MHT CET - 2025
- Gravitational Potential Energy
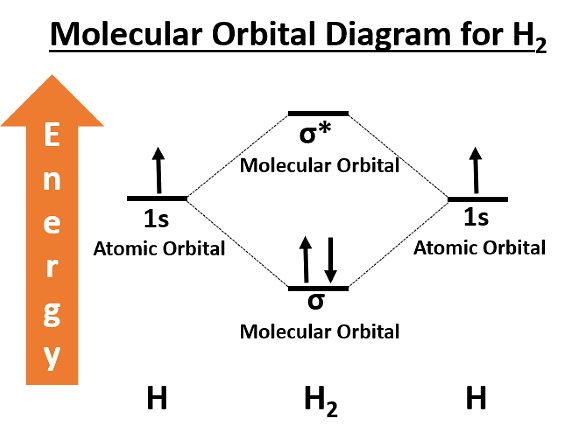
Concepts Used:
Molecular Orbital Theory
The Molecular Orbital Theory is a more sophisticated model of chemical bonding where new molecular orbitals are generated using a mathematical process called Linear Combination of Atomic Orbitals (LCAO).
Molecular Orbital theory is a chemical bonding theory that states that individual atoms combine together to form molecular orbitals. Due to this arrangement in MOT Theory, electrons associated with different nuclei can be found in different atomic orbitals. In molecular orbital theory, the electrons present in a molecule are not assigned to individual chemical bonds between the atoms. Rather, they are treated as moving under the influence of the atomic nuclei in the entire molecule.