Draw diagrams showing the formation of a double bond and a triple bond between carbon atoms in \(C_2H_4\) and \(C_2H_2\) molecules.
Solution and Explanation
\(C_2H_4\) :
The electronic configuration of \(C-atom\) in the excited state is:
\(_6C\)=\(1s^2 2s^1 2p_x^1 2p_y^1 2p_z^1\)
In the formation of an ethane molecule \((C_2H_4)\), one \(sp ^2\) hybrid orbital of carbon overlaps a \(sp ^2\) hybridized orbital of another carbon atom, thereby forming a \(C-C\) sigma bond.
The remaining two \(sp ^2\) orbitals of each carbon atom form a \(sp ^2\) \(-s\) sigma bond with two hydrogen atoms. The unhybridized orbital of one carbon atom undergoes sidewise overlap with the orbital of a similar kind present on another carbon atom to form a weak \(\pi-bond\).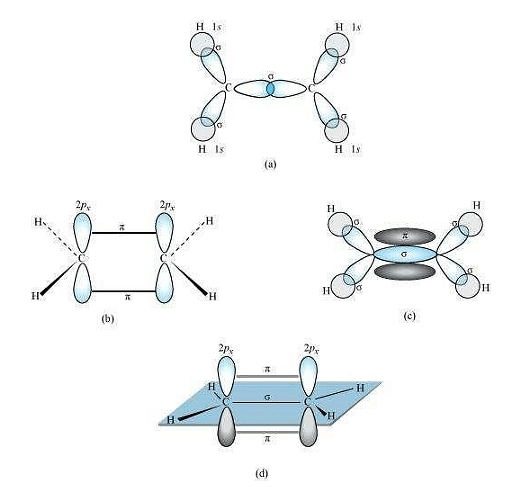
\(C_2H_2\):
In the formation of \(C_2H_2\) molecule, each \(C-atom\) is \(sp\) hybridized with two \(2p-orbitals\) in an unhybridized state.
One \(sp\) orbital of each carbon atom overlaps with the other along the internuclear axis forming a \(C-C \;sigma\) bond. The second \(sp\) orbital of each \(C-atom\) overlaps a half-filled \(1s-orbital\) to form a \(\sigma\) bond.
The two unhybridized \(2p-orbitals\) of the first carbon undergo sidewise overlap with the \(2p\; orbital\) of another carbon atom, thereby forming two \(pi (\pi)\) bonds between carbon atoms.
Hence, the triple bond between two carbon atoms is made up of one sigma and two \(\pi-bonds\).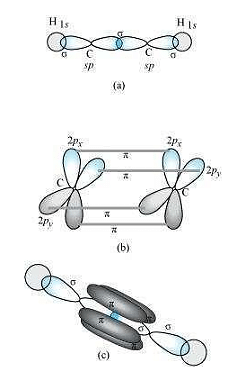
Top Questions on Bond Parameters
Match List-I with List-II and select the correct option:

- KCET - 2025
- Chemistry
- Bond Parameters
- The correct statement/s about Hydrogen bonding is/are :
A. Hydrogen bonding exists when H is covalently bonded to the highly electro negative atom.
B. Intermolecular H bonding is present in o-nitro phenol
C. Intramolecular H bonding is present in HF.
D. The magnitude of H bonding depends on the physical state of the compound.
E. H-bonding has powerful effect on the structure and properties of compounds.
Choose the correct answer from the options given below :- JEE Main - 2024
- Chemistry
- Bond Parameters
- How many of the following compounds have zero dipole moment ?
\(NH_3, H_2O, HF, CO_2, SO_2, BF_3, CH_4\)- JEE Main - 2024
- Chemistry
- Bond Parameters
- The correct order of bond angles of the molecules \( SiCl_4 \), \( SO_3 \), \( NH_3 \), \( HgCl_2 \) is:
- AP EAPCET - 2024
- Chemistry
- Bond Parameters
The bond angles \( b_1, b_2, b_3 \) in the above structure are respectively in \( ^\circ \):

- AP EAPCET - 2024
- Chemistry
- Bond Parameters
Questions Asked in CBSE Class XI exam
- Balance the following redox reactions by ion-electron method:
(a)MnO4- (aq) + I - (aq) → MnO2(s) + I2(s) (in basic medium)
(b) MnO4- (aq) + SO2(g) → Mn2+(aq) +HSO4- (aq) (in acidic solution)
(c) H2O2(aq)+Fe2+(aq) → Fe3+ (aq) + H2O (l) (in acidic solution)
(d) Cr2O72-+ SO2(g) → Cr3+ (aq) +SO42- (aq) (in acidic solution)- CBSE Class XI
- Oxidation Number
- Write the resonance structures for SO3 , NO2 and NO3-
- CBSE Class XI
- Kossel-Lewis Approach to Chemical Bonding
- At 700 K, equilibrium constant for the reaction:
\(H_2 (g) + I_2 (g) ⇋ 2HI (g)\)
is 54.8. If 0.5 mol L–1 of HI(g) is present at equilibrium at 700 K, what are the concentration of H2(g) and I2(g) assuming that we initially started with HI(g) and allowed it to reach equilibrium at 700 K?- CBSE Class XI
- Law Of Chemical Equilibrium And Equilibrium Constant
- Find the mean deviation about the mean for the data 4, 7, 8, 9, 10, 12, 13, 17.
- CBSE Class XI
- Statistics
Find the mean deviation about the mean for the data 38, 70, 48, 40, 42, 55, 63, 46, 54, 44.
- CBSE Class XI
- Statistics
Concepts Used:
Chemical Bonding and Molecular Structure
Such a group of atoms is called a molecule. Obviously, there must be some force that holds these constituent atoms together in the molecules. The attractive force which holds various constituents (atoms, ions, etc.) together in different chemical species is called a chemical bond.
Types of Chemical Bonds:
There are 4 types of chemical bonds which are formed by atoms or molecules to yield compounds.
- Ionic Bonds - Ionic bonding is a type of chemical bonding which involves a transfer of electrons from one atom or molecule to another.
- Covalent Bonds - Compounds that contain carbon commonly exhibit this type of chemical bonding.
- Hydrogen Bonds - It is a type of polar covalent bonding between oxygen and hydrogen wherein the hydrogen develops a partial positive charge
- Polar Bonds - In Polar Covalent chemical bonding, electrons are shared unequally since the more electronegative atom pulls the electron pair closer to itself and away from the less electronegative atom.
Factors Affecting Bond Enthalpy in Chemical Bonding:
- Size of the Atom
- Multiplicity of Bonds
- Number of Lone Pair of Electrons Present
- Bond Angle