Write down the IUPAC name for each of the following complexes and indicate the oxidation state, electronic configuration and coordination number. Also give stereochemistry and magnetic moment of the complex:
\((i) K[Cr(H_2O)_2(C_2O_4)_2].3H_2O\)
\((ii)[Co(NH_3)_5Cl]Cl_2\)
\((iii) CrCl_3(py)_3\)
\((iv) Cs[FeCl_4]\)
\((v)K_4[Mn(CN)_6]\)
Write down the IUPAC name for each of the following complexes and indicate the oxidation state, electronic configuration and coordination number. Also give stereochemistry and magnetic moment of the complex:
\((i) K[Cr(H_2O)_2(C_2O_4)_2].3H_2O\)
\((ii)[Co(NH_3)_5Cl]Cl_2\)
\((iii) CrCl_3(py)_3\)
\((iv) Cs[FeCl_4]\)
\((v)K_4[Mn(CN)_6]\)
Solution and Explanation
(i) Potassium diaquadioxalatochromate (III) trihydrate.
Oxidation state of chromium=3
Electronic configuration: \(3d ^{3} : t_{2g}^ 3 \)
Coordination number = 6 Shape: octahedral
Stereochemistry: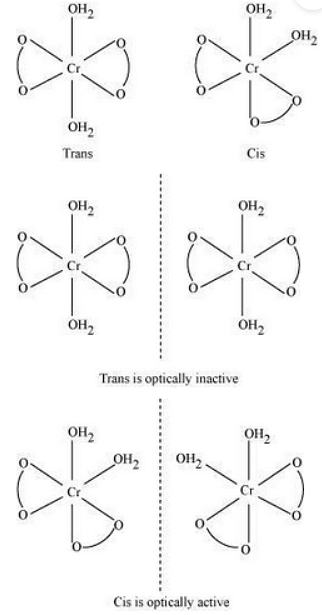
Magnetic moment, \(ÃŽÂ\frac{1}{4}=\sqrt{n(n+2)}\)
\(=\sqrt{3(3+2)}\)
\(=\sqrt{15}\)
\(∼ 4BM\)
\((ii) [Co(NH_3)_5Cl]Cl_2 \)
IUPAC name: Pentaamminechloridocobalt(III) chloride
Oxidation state of \( Co=+3 \)
Coordination number=6 Shape: octahedral.
Electronic configuration: \(d ^{6} : t_{2g}^ 6 .\)
Stereochemistry:

Magnetic Moment=0
\((iii) CrCl_3(py)_3 \)
IUPAC name: Trichloridotripyridinechromium (III)
Oxidation state of chromium = +3
Electronic configuration for \(d^{ 3} = t_{2g}^ 3 \)
Coordination number = 6
Shape: octahedral.
Stereochemistry:
Both isomers are optically active.
Therefore, a total of 4 isomers exist.
Magnetic moment\(, ÃŽÂ\frac{1}{4}=\sqrt{n(n+2)}\)
\(=\sqrt{3(3+2)}\)
\(=\sqrt{15}\)
∼ 4BM
\((iv) Cs[FeCl_4] \)
IUPAC name: Caesium tetrachloroferrate (III)
Oxidation state of\( Fe = +3\)
Electronic configuration of \(d ^{6} = eg^{ 2} t_{2g}^ 3 \)
Coordination number = 4 Shape: tetrahedral
Stereochemistry: optically inactive Magnetic moment: \(ÃŽÂ\frac{1}{4}=\sqrt{n(n+2)}\)
\(=\sqrt{5(5+2)}\)
\(=\sqrt{35}\)
~6BM
\((v) K_4[Mn(CN)_6]\) Potassium hexacyanomanganate(II)
Oxidation state of manganese=+2
Electronic configuration: \(d ^{5+}: t_{2g}^ 5 \)
Coordination number = 6 Shape: octahedral.
Streochemistry: optically inactive
Magnetic moment,\( ÃŽÂ\frac{1}{4}=\sqrt{n(n+2)}\)
\(=\sqrt{1(1+2)}\)
\(=\sqrt{3}\)
\(=1.732BM\)
Top Questions on Coordination Compounds
- Why are low spin tetrahedral complexes rarely observed?
- CBSE CLASS XII - 2025
- Chemistry
- Coordination Compounds
The correct IUPAC name of \([ \text{Pt}(\text{NH}_3)_2\text{Cl}_2 ]^{2+} \) is:
- CBSE CLASS XII - 2025
- Chemistry
- Coordination Compounds
- On the basis of \(\Delta_0\) and P (pairing energy), how can you differentiate between a strong field ligand and a weak field ligand?
- CBSE CLASS XII - 2025
- Chemistry
- Coordination Compounds
The Crystal Field Theory (CFT) of coordination compounds is based on the effect of different crystal fields (provided by the ligands taken as point charges) on the degeneracy of d-orbital energies of the central metal atom/ion. The splitting of the d-orbitals provides different electronic arrangements in strong and weak crystal fields. In tetrahedral coordination entity formation, the d-orbital splitting is smaller as compared to the octahedral entity.
What is crystal field splitting energy?- CBSE CLASS XII - 2025
- Chemistry
- Coordination Compounds
The Crystal Field Theory (CFT) of coordination compounds is based on the effect of different crystal fields (provided by the ligands taken as point charges) on the degeneracy of d-orbital energies of the central metal atom/ion. The splitting of the d-orbitals provides different electronic arrangements in strong and weak crystal fields. In tetrahedral coordination entity formation, the d-orbital splitting is smaller as compared to the octahedral entity.
On the basis of CFT, explain why [Ti(H$_2$O)$_6$]Cl$_3$ complex is coloured? What happens on heating the complex [Ti(H$_2$O)$_6$]Cl$_3$? Give reason.- CBSE CLASS XII - 2025
- Chemistry
- Coordination Compounds
Questions Asked in CBSE CLASS XII exam
If vector \( \mathbf{a} = 3 \hat{i} + 2 \hat{j} - \hat{k} \) \text{ and } \( \mathbf{b} = \hat{i} - \hat{j} + \hat{k} \), then which of the following is correct?
- Find the value of $x$, if \[ \begin{bmatrix} 1 & 3 & 2 \\ 2 & 5 & 1 \\ 15 & 3 & 2 \end{bmatrix} \begin{bmatrix} 1 \\ x \\ 2 \end{bmatrix} = \begin{bmatrix} 0 \\ 0 \\ 0 \end{bmatrix} \]
- Two point charges of \( -5\,\mu C \) and \( 2\,\mu C \) are located in free space at \( (-4\,\text{cm}, 0) \) and \( (6\,\text{cm}, 0) \) respectively.
(a) Calculate the amount of work done to separate the two charges at infinite distance.
(b) If this system of charges was initially kept in an electric field \[ \vec{E} = \frac{A}{r^2}, \text{ where } A = 8 \times 10^4\, \text{N}\,\text{C}^{-1}\,\text{m}^2, \] calculate the electrostatic potential energy of the system.- CBSE CLASS XII - 2025
- Electrostatics
- 4,000 shares of ₹ 10 each were forfeited for non-payment of second and final call money of ₹ 2 per share. The minimum amount that the company must collect at the time of reissue of these shares will be :
- CBSE CLASS XII - 2025
- Accounting for Share Capital
- If $y = a \cos(\log x) + b \sin(\log x)$, then $x^2y'' + xy'1$ is:
- CBSE CLASS XII - 2025
- Continuity and differentiability
Concepts Used:
Nomenclature of Coordination Compounds
Nomenclature of Coordination Compounds is important in Coordination Chemistry because of the need to have an unambiguous method of describing formulas and writing systematic names, particularly when dealing with isomers.
We can apply the following formulas:
- On the very first the central atom is listed.
- Ligands are then listed in alphabetical order and their placement in the list does not depend on their charge.
- Polydentate ligands are also listed alphabetically. In such a case of an abbreviated ligand, the first letter of the abbreviation is used to determine the position of the ligand in alphabetical order.
- The formula for the entire coordination entity is enclosed in square brackets whether charged or not. The formulas are enclosed in parentheses when ligands are polyatomic. Ligand abbreviations are also enclosed in parentheses.
- Within a coordination sphere, there should be no space between the ligands and the metal.
- When the formula of a charged coordination entity is to be written without that of the counter-ions, the charge is indicated outside the square brackets as a right superscript with the number before the sign. For example, [Co(CN)6]3-, [Cr(H2O)6]3+, etc.
- The charge of the anion(s) balances the charge of the cation(s).