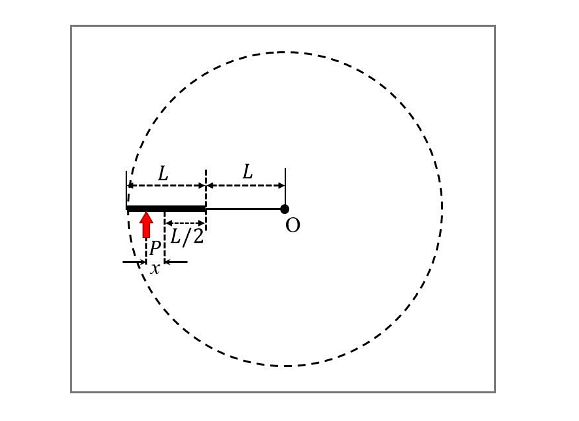
A thin uniform rod of length L and certain mass is kept on a frictionless horizontal table with a massless string of length L fixed to one end (top view is shown in the figure). The other end of the string is pivoted to a point O. If a horizontal impulse P is imparted to the rod at a distance x = L/n from the mid-point of the rod (see figure), then the rod and string revolve together around the point O, with the rod remaining aligned with the string. In such a case, the value of n is _____.

Solution and Explanation
Step 1: Define Angular Impulse \( I_A \)
\[ I_A = \text{angular impulse} = \vec{p} \times \vec{r} = \rho \left( \frac{l}{2} + x \right) \]
Step 2: Express Linear Impulse \( I \)
\[ I = \Delta p \]
Since \( p = mv - 0 \), we get:
\[ p = mv \]
Step 3: Write the Expression for \( I_A \)
\[ I_A = mv \times \left( \frac{3l}{2} + x \right) \]
Step 4: Define Angular Momentum \( L \)
\[ L = \text{angular momentum} = I_0 \omega + mv \times r \]
Substituting values:
\[ L = \frac{ml^2}{12} \omega + mv \times \frac{3l}{2} \]
Step 5: Relate Angular Impulse to Change in Angular Momentum
\[ mv \left( \frac{3l}{2} + x \right) = \frac{ml^2}{12} \omega + mv \frac{3l}{2} \]
Step 6: Solve for \( v \)
\[ mvx = \frac{ml^2}{12} \omega \]
Solving for \( v \):
\[ v = \frac{3l\omega}{2} \]
Step 7: Solve for \( x \)
\[ m\omega \cdot \frac{3l}{2} x = \frac{ml^2}{12} \times \omega \]
Solving for \( x \):
\[ x = \frac{l}{18} = \frac{l}{n} \]
Final Answer:
\( n = 18 \)
Top Questions on Friction
- A block of mass 5 kg is placed on a frictionless surface and pushed with a force of 20 N at an angle of 30° to the horizontal. What is the acceleration of the block?
- A cubic block of mass $ m $ is sliding down on an inclined plane at $ 60^\circ $ with an acceleration of $ \frac{g}{2} $, the value of coefficient of kinetic friction is:
- A particle is in uniform circular motion. The equation of its trajectory is given by \( x = 2t^2 - 3t + 5 \), where \( x \) and \( y \) are in meters. The speed of the particle is 2 m/s. When the particle attains the lowest \( y \)-coordinate, the acceleration of the particle is (in \( \text{m/s}^2 \)):
- KCET - 2025
- Physics
- Friction
- The minimum force required to start pushing a body up a rough (having coefficient of friction \( \mu \)) inclined plane is \( F_1 \), while the minimum force needed to prevent it from sliding is \( F_2 \). If the inclined plane makes an angle \( \theta \) with the horizontal such that \( \tan\theta = 2\mu \), then the ratio \( \frac{F_1}{F_2} \) is:
- WBJEE - 2025
- Physics
- Friction
- There are two inclined surfaces of equal length inclined at an angle of \(45^\circ\) with the horizontal. One of them is rough and the other is perfectly smooth. A given body takes 2 times as much time to slide down on the rough surface than on the smooth surface. The coefficient of kinetic friction (\(\mu_k\)) between the object and the rough surface is close to :
Questions Asked in JEE Advanced exam
- Let $ x_0 $ be the real number such that $ e^{x_0} + x_0 = 0 $. For a given real number $ \alpha $, define $$ g(x) = \frac{3xe^x + 3x - \alpha e^x - \alpha x}{3(e^x + 1)} $$ for all real numbers $ x $. Then which one of the following statements is TRUE?
- JEE Advanced - 2025
- Fundamental Theorem of Calculus
- A linear octasaccharide (molar mass = 1024 g mol$^{-1}$) on complete hydrolysis produces three monosaccharides: ribose, 2-deoxyribose and glucose. The amount of 2-deoxyribose formed is 58.26 % (w/w) of the total amount of the monosaccharides produced in the hydrolyzed products. The number of ribose unit(s) present in one molecule of octasaccharide is _____.
Use: Molar mass (in g mol$^{-1}$): ribose = 150, 2-deoxyribose = 134, glucose = 180; Atomic mass (in amu): H = 1, O = 16- JEE Advanced - 2025
- Biomolecules
Let $ P(x_1, y_1) $ and $ Q(x_2, y_2) $ be two distinct points on the ellipse $$ \frac{x^2}{9} + \frac{y^2}{4} = 1 $$ such that $ y_1 > 0 $, and $ y_2 > 0 $. Let $ C $ denote the circle $ x^2 + y^2 = 9 $, and $ M $ be the point $ (3, 0) $. Suppose the line $ x = x_1 $ intersects $ C $ at $ R $, and the line $ x = x_2 $ intersects $ C $ at $ S $, such that the $ y $-coordinates of $ R $ and $ S $ are positive. Let $ \angle ROM = \frac{\pi}{6} $ and $ \angle SOM = \frac{\pi}{3} $, where $ O $ denotes the origin $ (0, 0) $. Let $ |XY| $ denote the length of the line segment $ XY $. Then which of the following statements is (are) TRUE?
- JEE Advanced - 2025
- Conic sections
- Adsorption of phenol from its aqueous solution on to fly ash obeys Freundlich isotherm. At a given temperature, from 10 mg g$^{-1}$ and 16 mg g$^{-1}$ aqueous phenol solutions, the concentrations of adsorbed phenol are measured to be 4 mg g$^{-1}$ and 10 mg g$^{-1}$, respectively. At this temperature, the concentration (in mg g$^{-1}$) of adsorbed phenol from 20 mg g$^{-1}$ aqueous solution of phenol will be ____. Use: $\log_{10} 2 = 0.3$
- JEE Advanced - 2025
- Adsorption
- At 300 K, an ideal dilute solution of a macromolecule exerts osmotic pressure that is expressed in terms of the height (h) of the solution (density = 1.00 g cm$^{-3}$) where h is equal to 2.00 cm. If the concentration of the dilute solution of the macromolecule is 2.00 g dm$^{-3}$, the molar mass of the macromolecule is calculated to be $X \times 10^{4}$ g mol$^{-1}$. The value of $X$ is ____. Use: Universal gas constant (R) = 8.3 J K$^{-1}$ mol$^{-1}$ and acceleration due to gravity (g) = 10 m s$^{-2}\}$
- JEE Advanced - 2025
- Colligative Properties