A given quantity of gas is taken from A to C in two ways; a) directly from A\(\rightarrow\)C along a straight line and b) in two steps, from A\(\rightarrow\)B and then from B\(\rightarrow\)C.Work done and heat absorbed along the direct path A\(\rightarrow\)C are 200J and 280J respectively. If the work done along A\(\rightarrow\)B\(\rightarrow\)C is 80J, then heat absorbed along the path is,
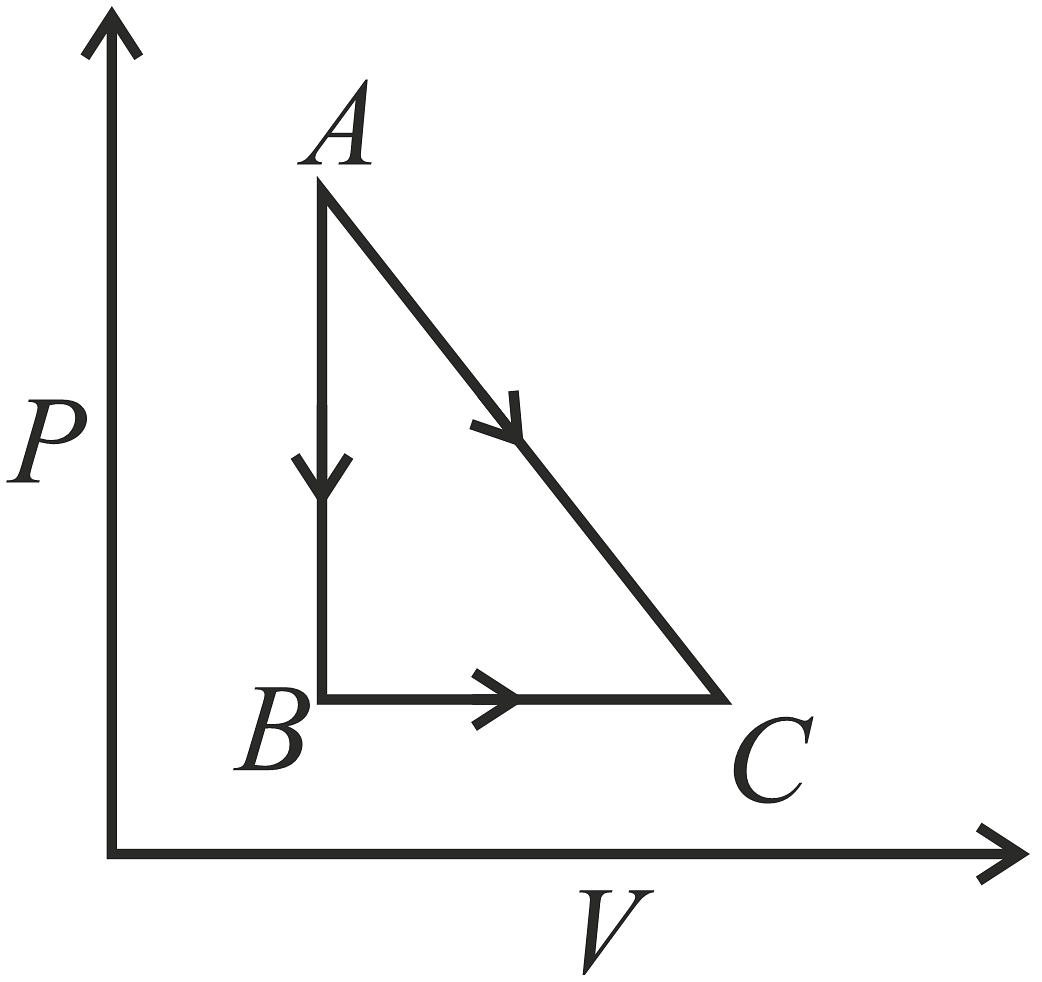

- 80J
- 0
- 160J
- 120J
The Correct Option is C
Approach Solution - 1
To determine the heat absorbed along the path A → B → C, let's break down the solution step by step:
Given Data:
- Work done along the direct path A → C: WAC = 200 J
- Heat absorbed along the direct path A → C: QAC = 280 J
- Work done along the path A → B → C: WABC = 80 J
Using the first law of thermodynamics for path A → C:
\[ \Delta U = Q_{AC} - W_{AC} = 280 - 200 = 80\,\text{J} \]
Since internal energy is a state function, ΔU is the same for both paths A → C and A → B → C.
Now, for path A → B → C:
\[ \Delta U = Q_{ABC} - W_{ABC} \]
\[ 80 = Q_{ABC} - 80 \Rightarrow Q_{ABC} = 160\,\text{J} \]
Conclusion: The heat absorbed along the path A → B → C is 160 J.
The correct option is (C): 160 J
Approach Solution -2
To understand the heat absorbed along path A → B → C in a thermodynamic process, we apply the First Law of Thermodynamics, which states:
ΔU = Q - W
Where:
- ΔU is the change in internal energy of the system
- Q is the heat added to the system
- W is the work done by the system
Step 1: Apply the first law to path A → C
From the question, along path A → C:
- Heat added: QAC = 300 J
- Work done: WAC = 200 J
Using the formula:
ΔU = Q - W = 300 - 200 = 100 J
This means that the internal energy increased by 100 J during the process. Since internal energy is a state function, this value of ΔU remains the same regardless of the path taken between states A and C.
Step 2: Apply the first law to path A → B → C
Now consider the alternate path A → B → C:
- Work done: WABC = 60 J
- We already know: ΔU = 100 J
Rearranging the first law to solve for heat:
Q = ΔU + W = 100 + 60 = 160 J
Conclusion: The heat absorbed along the path A → B → C is 160 J. This is less than the heat absorbed along path A → C because less work is done in path A → B → C.
The correct option is (C): 160 J
Top Questions on Thermodynamics
- A cylindrical conductor of length \(2 \, \text{m}\) and area of cross-section \(0.2 \, \text{mm}^2\) carries an electric current of \(1.6 \, \text{A}\) when its ends are connected to a \(2 \, \text{V}\) battery. Mobility of electrons in the conductor is \( \alpha \times 10^{-3} \, \text{m}^2\text{/V s}. \) The value of \( \alpha \) is ______________.
(Electron concentration \(= 5 \times 10^{28} \, \text{m}^{-3}\), electron charge \(= 1.6 \times 10^{-19} \, \text{C}\))- JEE Main - 2026
- Physics
- Thermodynamics
- An insulated cylinder of volume \(60\,\text{cm}^3\) is filled with a gas at \(27^\circ\text{C}\) and \(2\) atmospheric pressure. The gas is then compressed making the final volume \(20\,\text{cm}^3\) while allowing the temperature to rise to \(77^\circ\text{C}\). The final pressure is ____________ atmospheric pressure.
- JEE Main - 2026
- Physics
- Thermodynamics
- In a Young's double slit experiment set up, the two slits are kept 0.4 mm apart and screen is placed at 1 m from slits. If a thin transparent sheet of thickness 20 $\mu$m is introduced in front of one of the slits then center bright fringe shifts by 20 mm on the screen. The refractive index of transparent sheet is given by $\frac{\alpha{10}$, where $\alpha$ is _________}.
- JEE Main - 2026
- Physics
- Thermodynamics
- Which of the following solutions is the most acidic?
- LPUNEST - 2026
- Chemistry
- Thermodynamics
- Match List - I with List - II. List - I (Partial Derivatives) \(and\) List - II (Thermodynamic Quantity)

In the light of the above statements, choose the correct answer from the options given below:- JEE Main - 2025
- Chemistry
- Thermodynamics
Questions Asked in WBJEE exam
- Figure shows the graph of angle of deviation \( \delta \) versus angle of incidence \( i \) for a light ray striking a prism. The prism angle is

- WBJEE - 2025
- Refraction Through A Prism
- Ruma reached the metro station and found that the escalator was not working. She walked up the stationary escalator with velocity \( v_1 \) in time \( t_1 \). On another day, if she remains stationary on the escalator moving with velocity \( v_2 \), the escalator takes her up in time \( t_2 \). The time taken by her to walk up with velocity \( v_1 \) on the moving escalator will be:
- WBJEE - 2025
- Relative Motion
- The compound(s) showing optical activity is/are
- WBJEE - 2025
- Stoichiometry and Stoichiometric Calculations
Which of the following statement(s) is/are correct about the given compound?

- WBJEE - 2025
- Organic Chemistry
Identify 'P' and 'Q' in the following reaction

- WBJEE - 2025
- Organic Reactions
Concepts Used:
Thermodynamics
Thermodynamics in physics is a branch that deals with heat, work and temperature, and their relation to energy, radiation and physical properties of matter.
Important Terms
System
A thermodynamic system is a specific portion of matter with a definite boundary on which our attention is focused. The system boundary may be real or imaginary, fixed or deformable.
There are three types of systems:
- Isolated System – An isolated system cannot exchange both energy and mass with its surroundings. The universe is considered an isolated system.
- Closed System – Across the boundary of the closed system, the transfer of energy takes place but the transfer of mass doesn’t take place. Refrigerators and compression of gas in the piston-cylinder assembly are examples of closed systems.
- Open System – In an open system, the mass and energy both may be transferred between the system and surroundings. A steam turbine is an example of an open system.
Thermodynamic Process
A system undergoes a thermodynamic process when there is some energetic change within the system that is associated with changes in pressure, volume and internal energy.
There are four types of thermodynamic process that have their unique properties, and they are:
- Adiabatic Process – A process in which no heat transfer takes place.
- Isochoric Process – A thermodynamic process taking place at constant volume is known as the isochoric process.
- Isobaric Process – A process in which no change in pressure occurs.
- Isothermal Process – A process in which no change in temperature occurs.
Laws of Thermodynamics
Zeroth Law of Thermodynamics
The Zeroth law of thermodynamics states that if two bodies are individually in equilibrium with a separate third body, then the first two bodies are also in thermal equilibrium with each other.
First Law of Thermodynamics
The First law of thermodynamics is a version of the law of conservation of energy, adapted for thermodynamic processes, distinguishing three kinds of transfer of energy, as heat, as thermodynamic work, and as energy associated with matter transfer, and relating them to a function of a body's state, called internal energy.
Second Law of Thermodynamics
The Second law of thermodynamics is a physical law of thermodynamics about heat and loss in its conversion.
Third Law of Thermodynamics
Third law of thermodynamics states, regarding the properties of closed systems in thermodynamic equilibrium: The entropy of a system approaches a constant value when its temperature approaches absolute zero.