A cyclic process is shown in the p-v diagram and T-S diagram. Which of the following statement(s) is/are true?
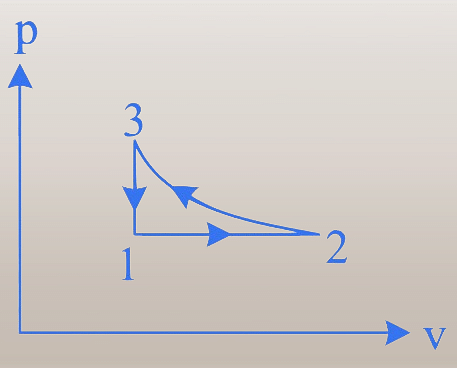
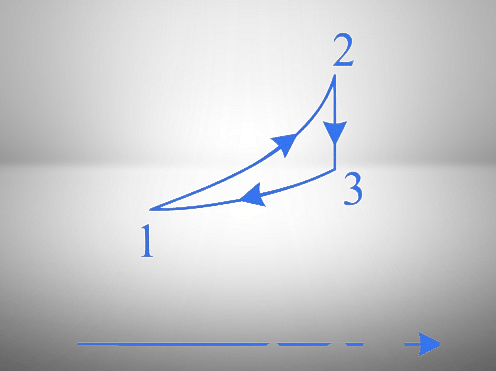


- 1\(\rightarrow\)2: Isobaric,2\(\rightarrow\)3:Isothermal
- 3\(\rightarrow\)1:Isochoric,2\(\rightarrow\)3:adiabatic
- Work done by the system in the complete cyclic process in non-zero.
- The heat absorbed by the system in the complete cyclic process is non-zero.
The Correct Option is B, C, D
Solution and Explanation
To solve the given problem, we need to analyze the cyclic process shown in both the $p$-$v$ and $T$-$S$ diagrams.
Analyzing the Diagrams
$p$-$v$ Diagram:
1. Process 1 → 2: The curve shows a decrease in pressure and an increase in volume.
2. Process 2 → 3: The curve shows a decrease in both pressure and volume.
3. Process 3 → 1: The curve shows an increase in pressure with a constant volume, indicating an isochoric process.
$T$-$S$ Diagram:
1. Process 1 → 2: The curve shows an increase in both temperature and entropy.
2. Process 2 → 3: The curve shows a decrease in temperature with constant entropy, indicating an adiabatic process.
3. Process 3 → 1: The curve shows a decrease in entropy and temperature.
Validating the Statements
Statement (B):
3 → 1: Isochoric, 2 → 3: Adiabatic
- 3 → 1: In the $p$-$v$ diagram, volume is constant while pressure changes, confirming an isochoric process.
- 2 → 3: In the $T$-$S$ diagram, entropy is constant while temperature changes, confirming an adiabatic process.
Thus, Statement (B) is correct.
Statement (C):
Work done by the system in the complete cyclic process is non-zero
- In a cyclic process, the work done by the system is represented by the area enclosed by the cycle in the $p$-$v$ diagram. Since the process forms a closed loop, the enclosed area indicates non-zero work done.
Thus, Statement (C) is correct.
Statement (D):
The heat absorbed by the system in the complete cyclic process is non-zero
- According to the first law of thermodynamics for a cyclic process, the net change in internal energy over one complete cycle is zero ($\Delta U = 0$). This implies that the heat absorbed ($Q$) is equal to the work done ($W$). Since the work done is non-zero (as confirmed in Statement (C)), the heat absorbed must also be non-zero.
Thus, Statement (D) is correct.
Conclusion:
The correct statements are:
(B) 3 → 1: Isochoric, 2 → 3: Adiabatic
(C) Work done by the system in the complete cyclic process is non-zero
(D) The heat absorbed by the system in the complete cyclic process is non-zero
Questions Asked in WBJEE exam
- Figure shows the graph of angle of deviation \( \delta \) versus angle of incidence \( i \) for a light ray striking a prism. The prism angle is

- WBJEE - 2025
- Refraction Through A Prism
- Ruma reached the metro station and found that the escalator was not working. She walked up the stationary escalator with velocity \( v_1 \) in time \( t_1 \). On another day, if she remains stationary on the escalator moving with velocity \( v_2 \), the escalator takes her up in time \( t_2 \). The time taken by her to walk up with velocity \( v_1 \) on the moving escalator will be:
- WBJEE - 2025
- Relative Motion
- The compound(s) showing optical activity is/are
- WBJEE - 2025
- Stoichiometry and Stoichiometric Calculations
Which of the following statement(s) is/are correct about the given compound?

- WBJEE - 2025
- Organic Chemistry
- X is an extensive property and x is an intensive property of a thermodynamic system. Which of the following statement(s) is (are) correct?
- WBJEE - 2025
- Thermodynamics
Concepts Used:
Thermodynamics
Thermodynamics in physics is a branch that deals with heat, work and temperature, and their relation to energy, radiation and physical properties of matter.
Important Terms
System
A thermodynamic system is a specific portion of matter with a definite boundary on which our attention is focused. The system boundary may be real or imaginary, fixed or deformable.
There are three types of systems:
- Isolated System – An isolated system cannot exchange both energy and mass with its surroundings. The universe is considered an isolated system.
- Closed System – Across the boundary of the closed system, the transfer of energy takes place but the transfer of mass doesn’t take place. Refrigerators and compression of gas in the piston-cylinder assembly are examples of closed systems.
- Open System – In an open system, the mass and energy both may be transferred between the system and surroundings. A steam turbine is an example of an open system.
Thermodynamic Process
A system undergoes a thermodynamic process when there is some energetic change within the system that is associated with changes in pressure, volume and internal energy.
There are four types of thermodynamic process that have their unique properties, and they are:
- Adiabatic Process – A process in which no heat transfer takes place.
- Isochoric Process – A thermodynamic process taking place at constant volume is known as the isochoric process.
- Isobaric Process – A process in which no change in pressure occurs.
- Isothermal Process – A process in which no change in temperature occurs.
Laws of Thermodynamics
Zeroth Law of Thermodynamics
The Zeroth law of thermodynamics states that if two bodies are individually in equilibrium with a separate third body, then the first two bodies are also in thermal equilibrium with each other.
First Law of Thermodynamics
The First law of thermodynamics is a version of the law of conservation of energy, adapted for thermodynamic processes, distinguishing three kinds of transfer of energy, as heat, as thermodynamic work, and as energy associated with matter transfer, and relating them to a function of a body's state, called internal energy.
Second Law of Thermodynamics
The Second law of thermodynamics is a physical law of thermodynamics about heat and loss in its conversion.
Third Law of Thermodynamics
Third law of thermodynamics states, regarding the properties of closed systems in thermodynamic equilibrium: The entropy of a system approaches a constant value when its temperature approaches absolute zero.