A compound gives two fragments in a mass spectrometer. They are C19H7N+ and C19H50+. To separate peaks due to these fragments the instrument should have a resolution of
- They cannot be separated
- 2.417 x 10-2
- 3.402 × 104
- 1.042 × 104
The Correct Option is D
Solution and Explanation
The mass spectrometer peaks given in the question are associated with two fragments, namely, C19H7N+ and C19H5O+. The goal is to determine the resolution required for the instrument to distinguish between these two peaks.
The resolution \(R\) in mass spectrometry is defined as:
\(R = \frac{m}{\Delta m}\)
where:
- \(m\) is the average mass of the two fragments.
- \(\Delta m\) is the difference in mass between the fragments.
First, calculate the mass of each fragment:
- For C19H7N+:
- Carbon (C): 19 atoms × 12.01 u = 228.19 u
- Hydrogen (H): 7 atoms × 1.008 u = 7.056 u
- Nitrogen (N): 1 atom × 14.01 u = 14.01 u
- Total mass = 228.19 + 7.056 + 14.01 = 249.256 u
- For C19H5O+:
- Carbon (C): 19 atoms × 12.01 u = 228.19 u
- Hydrogen (H): 5 atoms × 1.008 u = 5.04 u
- Oxygen (O): 1 atom × 16.00 u = 16.00 u
- Total mass = 228.19 + 5.04 + 16.00 = 249.23 u
The average mass, \(m\), is:
\(m = \frac{249.256 + 249.23}{2} = 249.243 u\)
The difference in mass, \(\Delta m\), is:
\(\Delta m = 249.256 - 249.23 = 0.026 u\)
Now, calculate the required resolution:
\(R = \frac{249.243}{0.026} \approx 9586.2692\)
The closest higher resolution given in the options, which allows for the separation, is \(1.042 \times 10^{4}\).
Therefore, the correct answer is:
1.042 × 104
Top Questions on Mass spectrometry
- The major product in the given reaction sequence is Q. The mass spectrum of Q shows
([M] = molecular ion peak)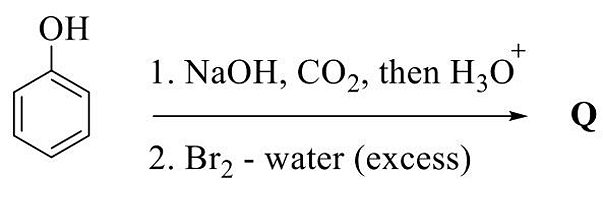
- GATE CY - 2024
- Inorganic Chemistry
- Mass spectrometry
- Recombination of electron-hole produces ____ in LEDs.
- AP PGECET - 2024
- Instrumentation Engineering
- Mass spectrometry
- Which among the following characteristics of Laser light specifies the precise movement of all individual light waves together through time and space?
- AP PGECET - 2024
- Instrumentation Engineering
- Mass spectrometry
- Magnetic sector analyzer is a part of
- AP PGECET - 2024
- Instrumentation Engineering
- Mass spectrometry
- Which ionization technique in mass spectrometry is most suitable for large biomolecules like proteins:
- GPAT - 2024
- Pharmaceutical Analysis
- Mass spectrometry
Questions Asked in GPAT exam
- Match the following:
(1) Schedule FF
(2) Schedule F3
(3) Schedule V
(4) Schedule Y
Descriptions:
(P) Standards of patent and proprietary medicines
(Q) Requirements and guidelines for clinical trials
(R) Standards for sterilized umbilical tapes
(S) Standards for Ophthalmic preparations- GPAT - 2025
- Drug therapy
- If the label or the container bears the name of an individual or company purporting to be the manufacturer of the drug, which individual or company is fictitious or does not exist, it is:
- GPAT - 2025
- Drug therapy
- Manufacturing Specification for tooling has been standardized by?
- GPAT - 2025
- Pharmacy Profession & Introduction to Pharmaceuticals
- As per USP, the maximum concentration of benzalkonium chloride used as a preservative in parenteral formulations is
- GPAT - 2025
- Pharmaceutical Analysis
Match the following:
(P) Schedule H
(Q) Schedule G
(R) Schedule P
(S) Schedule F2
Descriptions:
(I) Life period of drugs
(II) Drugs used under RMP
(III) List of Prescription Drugs
(IV) Standards for surgical dressing
- GPAT - 2025
- Drug therapy