Zr (Z=40) and Hf (Z=72) have similar atomic and ionic radii because of
having similar chemical properties
belonging to same group
diagonal relationship
lanthanoid contraction
The Correct Option is D
Solution and Explanation
To understand why Zr (Zirconium) and Hf (Hafnium) have similar atomic and ionic radii, we need to delve into the concept of "lanthanoid contraction".
- Understanding Lanthanoid Contraction:
- The lanthanoid contraction refers to the steady decrease in the size of the atoms and ions of the lanthanoids with increasing atomic number, from Lanthanum (La) to Lutetium (Lu).
- This is because as we move across the lanthanoid series, new electrons are added to the f-orbital, and the increased effective nuclear charge causes a greater attraction between the electrons and the nucleus, resulting in a smaller atomic and ionic size.
- Impact on Zirconium (Zr) and Hafnium (Hf):
- Zirconium and Hafnium are placed in the same group (Group 4) of the periodic table, thus having similar electronic configuration in their outermost shells.
- Despite the difference in atomic numbers (Zr = 40 and Hf = 72), their atomic and ionic radii are strikingly similar. This anomaly arises because of the lanthanoid contraction.
- The addition of 4f electrons in the lanthanoid series, which occurs just before Hafnium, causes a contraction that offsets the increase in size that would ordinarily be expected from the additional electron shells.
- Conclusion:
- Therefore, the similar atomic and ionic radii of Zr and Hf can be attributed directly to the lanthanoid contraction, which is highlighted in the correct option.
Hence, the correct answer is lanthanoid contraction.
Top Questions on d -and f -Block Elements
- At temperature \(T\) K, \(2\) moles of liquid \(A\) and \(3\) moles of liquid \(B\) are mixed. The vapour pressure of the ideal solution formed is \(320\) mm Hg. At this stage, one mole of \(A\) and one mole of \(B\) are added to the solution. The vapour pressure is now measured as \(328.6\) mm Hg. The vapour pressures (in mm Hg) of pure \(A\) and pure \(B\) respectively are:
- JEE Main - 2026
- Chemistry
- d -and f -Block Elements
- Consider the general reaction given below at 400 K: xA(g) ⇌ yB(g). The values of K___p and K___c are studied under the same condition of temperature but variation in x and y.
(i) K___p = 85.87 and K___c = 2.586
(ii) K___p = 0.862 and K___c = 28.62.
The values of x and y in (i) and (ii) respectively are :- JEE Main - 2026
- Chemistry
- d -and f -Block Elements
- Among the following oxides of 3d elements, the number of mixed oxides are ____________.
\[ \mathrm{Ti_2O_3,\ V_2O_4,\ Cr_2O_3,\ Mn_3O_4,\ Fe_3O_4,\ Fe_2O_3,\ Co_3O_4} \]- JEE Main - 2026
- Chemistry
- d -and f -Block Elements
- Statement-I : Consider the following pairs of ions $(\text{Sc}^{3+}, \text{Ti}^{3+}), (\text{Ti}^{4+}, \text{Ni}^{2+}), (\text{Cu}^{2+}, \text{Zn}^{2+})$ and $(\text{Cr}^{3+}, \text{Mn}^{3+})$. Out of these pairs three pairs consist of ions that are both coloured :
Statement-II : Among the lanthanide ions $\text{Eu}^{2+}$, $\text{Gd}^{3+}$, $\text{Ce}^{4+}$ and $\text{Tb}^{4+}$, the ion $\text{Tb}^{4+}$ is the strongest reducing agent.
Choose the correct option.- JEE Main - 2026
- Chemistry
- d -and f -Block Elements
- Why is the ability of oxygen greater than fluorine to stabilise higher oxidation states of transition metals?
- CBSE CLASS XII - 2025
- Chemistry
- d -and f -Block Elements
Questions Asked in NEET exam
- Two cities X and Y are connected by a regular bus service with a bus leaving in either direction every T min. A girl is driving scooty with a speed of 60 km/h in the direction X to Y. She notices that a bus goes past her every 30 minutes in the direction of her motion, and every 10 minutes in the opposite direction. Choose the correct option for the period T of the bus service and the speed (assumed constant) of the buses.
- NEET (UG) - 2025
- Relative Velocity
- A physical quantity P is related to four observations a, b, c, and d as follows: P = a3 b2 (c / √d) The percentage errors of measurement in a, b, c, and d are 1%, 3%, 2%, and 4% respectively. The percentage error in the quantity P is:
- NEET (UG) - 2025
- Dimensional analysis and its applications
What is Microalbuminuria ?
- NEET (UG) - 2025
- Human physiology
The output (Y) of the given logic implementation is similar to the output of an/a …………. gate.

- NEET (UG) - 2025
- Logic gates
- An oxygen cylinder of volume 30 litre has 18.20 moles of oxygen. After some oxygen is withdrawn from the cylinder, its gauge pressure drops to 11 atmospheric pressure at temperature \(27^\circ\)C. The mass of the oxygen withdrawn from the cylinder is nearly equal to: [Given, \(R = \frac{100}{12} \text{ J mol}^{-1} \text{K}^{-1}\), and molecular mass of \(O_2 = 32 \text{ g/mol}\), 1 atm pressure = \(1.01 \times 10^5 \text{ N/m}^2\)]
- NEET (UG) - 2025
- Ideal-gas equation and absolute temperature
Concepts Used:
Lanthanoids
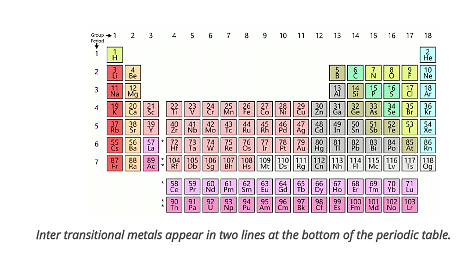
Lanthanoids are at the top of these two-row, while actinoids are at the bottom row.
Properties of Lanthanoids
Lanthanoids are inclusive of 14 elements, with atomic numbers 58-71:
- Cerium - Xe 4f1 5d1 6s2
- Praseodymium - Xe 4f3 6s2
- Neodymium - Xe 4f4 6s2
- Promethium - Xe 4f5 6s2
- Samarium - Xe 4f6 6s2
- Europium - Xe 4f7 6s2
- Gadolinium - Xe 4f7 5d1 6s2
- Terbium - Xe 4f9 6s2
- Dysprosium - Xe 4f10 6s2
- Holmium - Xe 4f11 6s2
- Erbium - Xe 4f12 6s2
- Thulium - Xe 4f13 6s2
- Ytterbium - Xe 4f14 6s2
- Lutetium - Xe 4f14 5d1 6s2
These elements are also called rare earth elements. They are found naturally on the earth, and they're all radioactively stable except promethium, which is radioactive. A trend is one of the interesting properties of the lanthanoid elements, called lanthanide contraction.