Question:
Zone refining is based on the solubility of impurities in the molten state than in the solid state of the metal.
Pure metal oxide is obtained in zone refining.
Zone refining is based on the solubility of impurities in the molten state than in the solid state of the metal.
Pure metal oxide is obtained in zone refining.
Updated On: Jul 28, 2022
- If both assertion and reason are true and reason is the correct explanation of assertion
- If both assertion and reason are true but reason is not the correct explanation of assertion
- If assertion is true but reason is false
- If both assertion and reason are false
Hide Solution
Verified By Collegedunia
The Correct Option is C
Solution and Explanation
Zone refining produces pure metal.
Was this answer helpful?
0
0
Top Questions on General Principles and Processes of Isolation of Elements
- What is used for the Thermite Reaction?
- BCECE - 2025
- Chemistry
- General Principles and Processes of Isolation of Elements
- In the extraction of iron using blast furnace to remove the impurity (X), chemical (Y) is added to the ore. X and Y are respectively
- TS EAMCET - 2025
- Chemistry
- General Principles and Processes of Isolation of Elements
- Which of the following compounds is used to cover the surface of a metallic object to prevent corrosion?
- KEAM - 2025
- Chemistry
- General Principles and Processes of Isolation of Elements
- The incorrect statement about the Hall-Heroult process is:
- KCET - 2024
- Chemistry
- General Principles and Processes of Isolation of Elements
- Select the correct statement:
- KCET - 2024
- Chemistry
- General Principles and Processes of Isolation of Elements
View More Questions
Questions Asked in AIIMS exam
- The element Neodymium (Nd) belongs to the 4f series. What is its atomic number?
- AIIMS - 2024
- Modern Periodic Law And The Present Form Of The Periodic Table
- The correct increasing order of energy of orbitals in a hydrogen atom is:
- AIIMS - 2024
- Atomic Structure
- Which of the following is a globular protein?
- AIIMS - 2024
- Biomolecules
- Given that the surface charge density on a sphere is 200 μC/m2, what is the electric field at the surface of the sphere?
- AIIMS - 2024
- Electrostatics
- Which of the following is a crystalline solid?
- AIIMS - 2024
- The solid state
View More Questions
Concepts Used:
General Principles and Processes of Isolation of Elements
What are Ores and Minerals?
Minerals are the naturally occurring, homogeneous inorganic solid substances. They are having a definite chemical composition and crystalline structure, hardness and color. For example, copper pyrite, calamine, etc.
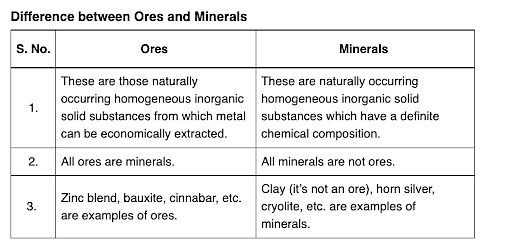
Impurities in an ore are called gauge. The removal of a gauge from the ore is called concentration ore.
Several steps are involved in the extraction of pure metal from ores. Major steps are as follows –
- Concentration of the ore
- Isolation of the metal from its concentrated ore
- Purification of the metal