Which one of the following has the same number of atoms as are in 6g of H2O
Which one of the following has the same number of atoms as are in 6g of H2O
0.4G He
22g CO2
1g H2
12g CO
The Correct Option is C
Solution and Explanation
To solve the problem, we need to find which option contains the same number of atoms as 6 g of H2O.
1. Calculate Moles of H2O:
Molar mass of water (H2O) = 18 g/mol
Moles of water = $ \frac{6}{18} = 0.333 \, \text{mol} $
2. Calculate Number of Atoms in H2O:
Each molecule of water contains 3 atoms (2 Hydrogen + 1 Oxygen).
So, total atoms = $0.333 \times 6.022 \times 10^{23} \times 3$
= $6.022 \times 10^{23} \times 1 = 6.022 \times 10^{23}$ atoms
3. Find Which Option Has the Same Number of Atoms:
- 1 g H2: Molar mass = 2 g/mol
Moles = $ \frac{1}{2} = 0.5 $ mol
Each H2 molecule has 2 atoms
Atoms = $0.5 \times 6.022 \times 10^{23} \times 2 = 6.022 \times 10^{23}$ - 0.4 g He: Molar mass = 4 g/mol → 0.1 mol → $0.1 \times 6.022 \times 10^{23}$ = fewer atoms
- 22 g CO2: Molar mass = 44 g/mol → 0.5 mol
Each CO2 has 3 atoms → $0.5 \times 3 \times 6.022 \times 10^{23}$ = $9.033 \times 10^{23}$ atoms - 12 g CO: Molar mass = 28 g/mol → $ \frac{12}{28} \approx 0.43 $ mol
Each CO has 2 atoms → $0.43 \times 2 \times 6.022 \times 10^{23} \approx 5.2 \times 10^{23}$ atoms
Final Answer:
The option with the same number of atoms as in 6 g of water is 1 g H2.
Top Questions on Law Of Chemical Equilibrium And Equilibrium Constant
- Consider the following gaseous equilibrium in a closed container of volume $V$ at temperature $T$:
P$_2$(g) + Q$_2$(g) $\rightleftharpoons$ 2PQ(g)
Initially, 2 moles each of P$_2$(g), Q$_2$(g) and PQ(g) are present at equilibrium. One mole each of P$_2$ and Q$_2$ are added. The number of moles of P$_2$, Q$_2$ and PQ at the new equilibrium respectively are
- JEE Main - 2026
- Chemistry
- Law Of Chemical Equilibrium And Equilibrium Constant
- $X_2(g) + Y_2(g) \rightleftharpoons 2Z(g)$. Equilibrium moles of $X_2, Y_2, Z$ are 3, 3, 9 mol (in 1 L). 10 mol of Z(g) is added. New equilibrium moles of Z(g) is ___. (Nearest integer)
- JEE Main - 2026
- Chemistry
- Law Of Chemical Equilibrium And Equilibrium Constant
- Observe the following equilibrium in a 1 L flask:
A(g) <=> B(g)
At temperature T (in K), the equilibrium concentrations of A and B are 0.5 M and 0.375 M respectively.
Then 0.1 moles of A is added into the flask and the system is heated to temperature T again to re-establish equilibrium.
The new equilibrium concentrations (in M) of A and B respectively are:- JEE Main - 2026
- Chemistry
- Law Of Chemical Equilibrium And Equilibrium Constant
- The plot of \(\log_{10}K\) vs \(\frac{1}{T}\) gives a straight line. The intercept and slope respectively are (where \(K\) is equilibrium constant).
- JEE Main - 2026
- Chemistry
- Law Of Chemical Equilibrium And Equilibrium Constant
- For the reaction \( \mathrm{A \rightleftharpoons B} \), the number of moles of A and B at equilibrium in a \(1\,\text{L}\) vessel are \(0.50\) and \(0.375\), respectively. If \(0.10\) mol of A is added further, determine the number of moles of A and B at the new equilibrium.
- JEE Main - 2026
- Chemistry
- Law Of Chemical Equilibrium And Equilibrium Constant
Questions Asked in TS EAMCET exam
- A body is projected vertically upward with an initial velocity of 40 m/s. Calculate the maximum height reached by the body. (Take \( g = 9.8 \, \text{m/s}^2 \))
- TS EAMCET - 2025
- Kinematics
- The properties required for a material to be used as the core of an electromagnet are:
- TS EAMCET - 2025
- Magnetism and matter
- Which of the following statements are correct?
- TS EAMCET - 2025
- Colligative Properties
- At 27$^\circ$C, 100 mL of 0.05 M Cu$^{2+}$ solution is added to 1 L of 0.1 M KI. Find [KI] in resultant solution.
- TS EAMCET - 2025
- Stoichiometry and Stoichiometric Calculations
- 4.0 g of a mixture containing Na₂CO₃ and NaHCO₃ is heated to 673K. Loss in mass of the mixture is found to be 0.62g. The percentage of sodium carbonate in the mixture is
- TS EAMCET - 2025
- Stoichiometry and Stoichiometric Calculations
Concepts Used:
Law of Chemical Equilibrium
Law of Chemical Equilibrium states that at a constant temperature, the rate of a chemical reaction is directly proportional to the product of the molar concentrations of the reactants each raised to a power equal to the corresponding stoichiometric coefficients as represented by the balanced chemical equation.
Let us consider a general reversible reaction;
A+B ↔ C+D
After some time, there is a reduction in reactants A and B and an accumulation of the products C and D. As a result, the rate of the forward reaction decreases and that of backward reaction increases.
Eventually, the two reactions occur at the same rate and a state of equilibrium is attained.
By applying the Law of Mass Action;
The rate of forward reaction;
Rf = Kf [A]a [B]b
The rate of backward reaction;
Rb = Kb [C]c [D]d
Where,
[A], [B], [C] and [D] are the concentrations of A, B, C and D at equilibrium respectively.
a, b, c, and d are the stoichiometric coefficients of A, B, C and D respectively.
Kf and Kb are the rate constants of forward and backward reactions.
However, at equilibrium,
Rate of forward reaction = Rate of backward reaction.
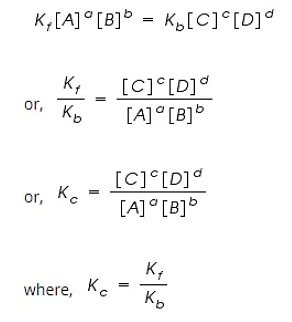
Kc is called the equilibrium constant expressed in terms of molar concentrations.
The above equation is known as the equation of Law of Chemical Equilibrium.