Which from following polymers is obtained using
Which from following polymers is obtained using
- Buna-S
- Polyacrylonitrile
- PVC
- Glyptal
The Correct Option is C
Solution and Explanation
Solution:
Understanding the Structure:
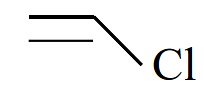
The structure is represented as follows:
CH2=CH-Cl
This is a vinyl chloride monomer, which consists of a vinyl group (–CH=CH2) attached to a chlorine atom. This monomer undergoes polymerization to form a polymer known as polyvinyl chloride (PVC).
Polyvinyl Chloride (PVC):
PVC is one of the most widely produced synthetic plastics. It is formed by the polymerization of vinyl chloride monomers. PVC is used in various applications such as pipes, flooring, medical devices, and electrical insulation.
Other Options:
Let’s briefly look at the other options to understand why they are not correct:
- Buna-S: Buna-S is a type of synthetic rubber made from the polymerization of styrene and butadiene, not vinyl chloride.
- Polyacrylonitrile: Polyacrylonitrile (PAN) is made from the polymerization of acrylonitrile monomers, not vinyl chloride.
- Glyptal: Glyptal is a polymer formed from the condensation of phthalic acid and glycerol, not vinyl chloride.
Conclusion:
The polymer obtained from the monomer shown in the image is Polyvinyl Chloride (PVC). Therefore, the correct answer is:
Option 3: PVC
Top Questions on Polymers
- A polymer melt being extruded through a spinneret hole of circular cross-section exhibits a die-swell ratio of 2.2. The extrusion velocity and volumetric flow rate during this extrusion process are 20 m/min and \(2.6 \times 10^{-8}\) m\(^3\)/s, respectively. The maximum diameter (mm) of the extruded melt after exit from the spinneret hole (rounded off to 2 decimal places) is:
- The main constituent of vinegar is:
- The monomer of natural rubber is:
- The catalyst used in the preparation of high density polythene is
- Arrange the following polymers in increasing order of intermolecular forces:

Questions Asked in MHT CET exam
Which part of root absorb mineral?
- MHT CET - 2025
- The Root
- A body of mass 2 kg is moving in a circular path of radius 3 m with a constant speed of 6 m/s. What is the centripetal force acting on the body?
- MHT CET - 2025
- Centripetal forces
- A 200 g sample of water at 80°C is mixed with 100 g of water at 20°C. Assuming no heat loss to the surroundings, what is the final temperature of the mixture?
- MHT CET - 2025
- thermal properties of matter
- Given the equation: \[ 81 \sin^2 x + 81 \cos^2 x = 30 \] Find the value of \( x \).
- MHT CET - 2025
- Trigonometric Identities
- A body of mass 10 kg is at a height of 5 m above the surface of the Earth. What is the gravitational potential energy of the body? (Take \( g = 10 \, \text{m/s}^2 \))
- MHT CET - 2025
- Gravitational Potential Energy
Concepts Used:
Types of Polymerization Reactions
Polymerization is a chemical reaction in which a large number of monomer molecules combine to produce a polymer. A polymerization can yield macromolecules with a linear or branching structure. They can also take the form of a three-dimensional complicated network.
Types of Polymerisation
Polymerization reactions are divided into two groups, namely, Addition polymerization (chain reaction) and condensation reaction (step reaction).
- Addition Polymerisation: As the name denotes, we can see the addition of monomers to form a polymer, twice the number or even more than that. Here, we can either select monomers of the same species or of different species. When we choose the single species monomers for the polymerization, the product thus formed is known as homopolymer (for example polythene used to make bags). When we use two different species for additional polymerization, the polymer would be known as copolymer (for example, Buna-S and Buna-N).
- Condensation Polymerisation: In the condensation polymerisation, we can find elimination of compounds like HCL, water, alcohol etc...; while the monomers condense during the reaction process. This reaction happens between tri-function and bi-function monomer units and results in the formation of polymers like nylon.