Question:
The shape of $XeF_{5}^{-}$ will be
The shape of $XeF_{5}^{-}$ will be
Updated On: Jun 18, 2022
- Square pyramid
- Trigonal bipyramidal
- Planar
- Pentagonal bipyramid
Hide Solution
Verified By Collegedunia
The Correct Option is C
Solution and Explanation
Shape of $X e F _{5}^{-} \rightarrow$
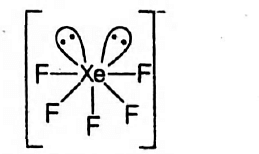
The Xe show $s p^{3} d^{3}$-hybridisation in $XeF _{5}^{-}$,
Thus, its geometry is Pentagonal-bipyramidal.
But, number of electron pair $=\frac{8+5+1}{2}=7$
Number of bond pair $=5^{2}$, number of lone pair $= 2$ .
A lot of electrons are present at axil position and all bonds are in same plane.
Hence, the shape is planar.

The Xe show $s p^{3} d^{3}$-hybridisation in $XeF _{5}^{-}$,
Thus, its geometry is Pentagonal-bipyramidal.
But, number of electron pair $=\frac{8+5+1}{2}=7$
Number of bond pair $=5^{2}$, number of lone pair $= 2$ .
A lot of electrons are present at axil position and all bonds are in same plane.
Hence, the shape is planar.
Was this answer helpful?
0
0
Top Questions on p -Block Elements
Method used for separation of mixture of products (B and C) obtained in the following reaction is:

- JEE Main - 2026
- Chemistry
- p -Block Elements
- Statement-I: An element ‘X’ of P-block forms a hydride H–X, which has the longest bond length, then element ‘X’ will have the shortest covalent radius.
Statement-II: An element ‘E’ of Group 15 forms hydride EH$_3$, that has least B.P. The maximum covalency of E is 4.
- JEE Main - 2026
- Chemistry
- p -Block Elements
- Given below are two statements:
Statement I: [CoBr₄]²⁻ ion will absorb light of lower energy than [CoCl₄]²⁻ ion.
Statement II: In [CoBr₄]²⁻ ion, the energy separation between the two set of d-orbitals is more than [CoCl₄]²⁻ ion.
In the light of the above statements, choose the correct answer from the options given below :- JEE Main - 2026
- Chemistry
- p -Block Elements
- Given below are two statements: Statement I: Elements \(X\) and \(Y\) are the most and least electronegative elements, respectively, among \(N\), \(As\), \(Sb\) and \(P\). The nature of the oxides \(X_2O_3\) and \(Y_2O_3\) is acidic and amphoteric, respectively. Statement II: \(BCl_3\) is covalent in nature and gets hydrolysed in water. It produces \([B(OH)_4]^-\) and \([B(H_2O)_6]^{3+}\) in aqueous medium. In the light of the above statements, choose the correct answer from the options given below:
- JEE Main - 2026
- Chemistry
- p -Block Elements
- For \( P \), the incorrect statement is:
- JEE Main - 2026
- Chemistry
- p -Block Elements
View More Questions
Questions Asked in WBJEE exam
- Figure shows the graph of angle of deviation \( \delta \) versus angle of incidence \( i \) for a light ray striking a prism. The prism angle is

- WBJEE - 2025
- Refraction Through A Prism
- Ruma reached the metro station and found that the escalator was not working. She walked up the stationary escalator with velocity \( v_1 \) in time \( t_1 \). On another day, if she remains stationary on the escalator moving with velocity \( v_2 \), the escalator takes her up in time \( t_2 \). The time taken by her to walk up with velocity \( v_1 \) on the moving escalator will be:
- WBJEE - 2025
- Relative Motion
- The compound(s) showing optical activity is/are
- WBJEE - 2025
- Stoichiometry and Stoichiometric Calculations
Which of the following statement(s) is/are correct about the given compound?

- WBJEE - 2025
- Organic Chemistry
- X is an extensive property and x is an intensive property of a thermodynamic system. Which of the following statement(s) is (are) correct?
- WBJEE - 2025
- Thermodynamics
View More Questions
Concepts Used:
P-Block Elements
- P block elements are those in which the last electron enters any of the three p-orbitals of their respective shells. Since a p-subshell has three degenerate p-orbitals each of which can accommodate two electrons, therefore in all there are six groups of p-block elements.
- P block elements are shiny and usually a good conductor of electricity and heat as they have a tendency to lose an electron. You will find some amazing properties of elements in a P-block element like gallium. It’s a metal that can melt in the palm of your hand. Silicon is also one of the most important metalloids of the p-block group as it is an important component of glass.
P block elements consist of:
- Group 13 Elements: Boron family
- Group 14 Elements: Carbon family
- Group 15 Elements: Nitrogen family
- Group 16 Elements: Oxygen family
- Group 17 Elements: Fluorine family
- Group 18 Elements: Neon family