Question:
The Kolbe's electrolysis proceeds via
The Kolbe's electrolysis proceeds via
Updated On: Jul 7, 2022
- Nucleophilic substitution mechanism
- Electrophilic addition mechanism
- Free radical mechanism
- Electrophilic substitution reaction
Hide Solution
Verified By Collegedunia
The Correct Option is C
Solution and Explanation
For example, when sodium propionate is electrolysed, $n$-butane. ethane; ethylene are obtained. The propionate ion discharges at the anode to form a free radical.
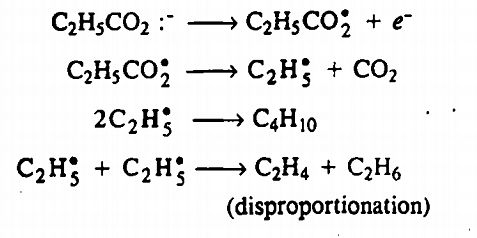
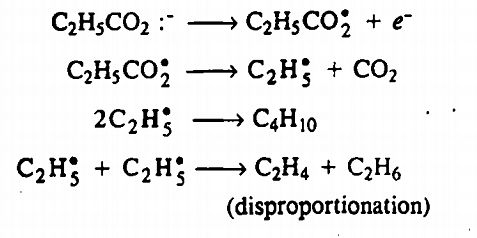
Was this answer helpful?
0
0
Top Questions on reaction mechanism
- Given below are two statements:
Statement I: The dipole moment of R–CN is greater than R–NC and R–NC can undergo hydrolysis under acidic medium to produce R–COOH.
Statement II: R–CN hydrolyses under acidic medium to produce a compound which on treatment with SOCl$_2$, followed by the addition of NH$_3$ gives another compound (X). This compound (X) on treatment with NaOCl/NaOH gives a product, that on treatment with CHCl$_3$/KOH/$\Delta$ produces R–NC.
In the light of the above statements, choose the correct answer from the options given below.
- JEE Main - 2026
- Chemistry
- reaction mechanism
- Given below are two statements:
Statement I: Phenol on treatment with \( \mathrm{CHCl_3/aq.\ KOH} \) under refluxing condition, followed by acidification produces p-hydroxy benzaldehyde as the major product and o-hydroxy benzaldehyde as the minor product.
Statement II: The mixture of p-hydroxybenzaldehyde and o-hydroxybenzaldehyde can be easily separated through steam distillation.
In the light of the above statements, choose the correct answer from the options given below- JEE Main - 2026
- Chemistry
- reaction mechanism
The correct order of the rate of reaction of the following reactants with nucleophile by \( \mathrm{S_N1} \) mechanism is:
(Given: Structures I and II are rigid)
- JEE Main - 2026
- Chemistry
- reaction mechanism
- Best reducing agent among the given ions are:
- JEE Main - 2024
- Chemistry
- reaction mechanism
- Match the following:
LIST I LIST II A Lyman I Near IR B Balmer II Far IR C Paschen III Visible D p-fund IV UV - JEE Main - 2024
- Chemistry
- reaction mechanism
View More Questions