Question:
The correct statement(s) about the oxoacids, $HClO_4$ and $HClO$, is(are)
The correct statement(s) about the oxoacids, $HClO_4$ and $HClO$, is(are)
Updated On: Jun 14, 2022
- The conjugate base of $HClO _{4}$ is weaker base than $H _{2} O$
- The central atom in both $HClO _{4}$ and $HClO$ is $sp ^{3}$ hybridized.
- $HClO _{4}$ is more acidic than $HClO$ because of the resonance stabilization of its anion.
- $HClO _{4}$ is formed in the reaction between $Cl _{2}$ and $H _{2} O$.
Hide Solution
Verified By Collegedunia
The Correct Option is C
Solution and Explanation
Acidic Nature : $HClO _{4}> HClO$
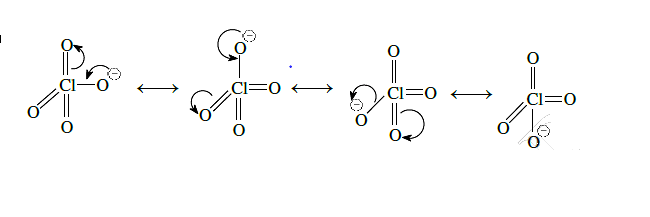
$H _{2} O + Cl _{2} \rightarrow HCl + HOCl$
 undefined
undefined
^

$H _{2} O + Cl _{2} \rightarrow HCl + HOCl$
 undefined
undefined
^
Was this answer helpful?
0
0
Top Questions on p -Block Elements
Method used for separation of mixture of products (B and C) obtained in the following reaction is:

- JEE Main - 2026
- Chemistry
- p -Block Elements
- Statement-I: An element ‘X’ of P-block forms a hydride H–X, which has the longest bond length, then element ‘X’ will have the shortest covalent radius.
Statement-II: An element ‘E’ of Group 15 forms hydride EH$_3$, that has least B.P. The maximum covalency of E is 4.
- JEE Main - 2026
- Chemistry
- p -Block Elements
- Given below are two statements:
Statement I: [CoBr₄]²⁻ ion will absorb light of lower energy than [CoCl₄]²⁻ ion.
Statement II: In [CoBr₄]²⁻ ion, the energy separation between the two set of d-orbitals is more than [CoCl₄]²⁻ ion.
In the light of the above statements, choose the correct answer from the options given below :- JEE Main - 2026
- Chemistry
- p -Block Elements
- Given below are two statements: Statement I: Elements \(X\) and \(Y\) are the most and least electronegative elements, respectively, among \(N\), \(As\), \(Sb\) and \(P\). The nature of the oxides \(X_2O_3\) and \(Y_2O_3\) is acidic and amphoteric, respectively. Statement II: \(BCl_3\) is covalent in nature and gets hydrolysed in water. It produces \([B(OH)_4]^-\) and \([B(H_2O)_6]^{3+}\) in aqueous medium. In the light of the above statements, choose the correct answer from the options given below:
- JEE Main - 2026
- Chemistry
- p -Block Elements
- For \( P \), the incorrect statement is:
- JEE Main - 2026
- Chemistry
- p -Block Elements
View More Questions
Questions Asked in JEE Advanced exam
- Let $ x_0 $ be the real number such that $ e^{x_0} + x_0 = 0 $. For a given real number $ \alpha $, define $$ g(x) = \frac{3xe^x + 3x - \alpha e^x - \alpha x}{3(e^x + 1)} $$ for all real numbers $ x $. Then which one of the following statements is TRUE?
- JEE Advanced - 2025
- Fundamental Theorem of Calculus
- A linear octasaccharide (molar mass = 1024 g mol$^{-1}$) on complete hydrolysis produces three monosaccharides: ribose, 2-deoxyribose and glucose. The amount of 2-deoxyribose formed is 58.26 % (w/w) of the total amount of the monosaccharides produced in the hydrolyzed products. The number of ribose unit(s) present in one molecule of octasaccharide is _____.
Use: Molar mass (in g mol$^{-1}$): ribose = 150, 2-deoxyribose = 134, glucose = 180; Atomic mass (in amu): H = 1, O = 16- JEE Advanced - 2025
- Biomolecules
Let $ P(x_1, y_1) $ and $ Q(x_2, y_2) $ be two distinct points on the ellipse $$ \frac{x^2}{9} + \frac{y^2}{4} = 1 $$ such that $ y_1 > 0 $, and $ y_2 > 0 $. Let $ C $ denote the circle $ x^2 + y^2 = 9 $, and $ M $ be the point $ (3, 0) $. Suppose the line $ x = x_1 $ intersects $ C $ at $ R $, and the line $ x = x_2 $ intersects $ C $ at $ S $, such that the $ y $-coordinates of $ R $ and $ S $ are positive. Let $ \angle ROM = \frac{\pi}{6} $ and $ \angle SOM = \frac{\pi}{3} $, where $ O $ denotes the origin $ (0, 0) $. Let $ |XY| $ denote the length of the line segment $ XY $. Then which of the following statements is (are) TRUE?
- JEE Advanced - 2025
- Conic sections
- Adsorption of phenol from its aqueous solution on to fly ash obeys Freundlich isotherm. At a given temperature, from 10 mg g$^{-1}$ and 16 mg g$^{-1}$ aqueous phenol solutions, the concentrations of adsorbed phenol are measured to be 4 mg g$^{-1}$ and 10 mg g$^{-1}$, respectively. At this temperature, the concentration (in mg g$^{-1}$) of adsorbed phenol from 20 mg g$^{-1}$ aqueous solution of phenol will be ____. Use: $\log_{10} 2 = 0.3$
- JEE Advanced - 2025
- Adsorption
- At 300 K, an ideal dilute solution of a macromolecule exerts osmotic pressure that is expressed in terms of the height (h) of the solution (density = 1.00 g cm$^{-3}$) where h is equal to 2.00 cm. If the concentration of the dilute solution of the macromolecule is 2.00 g dm$^{-3}$, the molar mass of the macromolecule is calculated to be $X \times 10^{4}$ g mol$^{-1}$. The value of $X$ is ____. Use: Universal gas constant (R) = 8.3 J K$^{-1}$ mol$^{-1}$ and acceleration due to gravity (g) = 10 m s$^{-2}\}$
- JEE Advanced - 2025
- Colligative Properties
View More Questions
Concepts Used:
P-Block Elements
- P block elements are those in which the last electron enters any of the three p-orbitals of their respective shells. Since a p-subshell has three degenerate p-orbitals each of which can accommodate two electrons, therefore in all there are six groups of p-block elements.
- P block elements are shiny and usually a good conductor of electricity and heat as they have a tendency to lose an electron. You will find some amazing properties of elements in a P-block element like gallium. It’s a metal that can melt in the palm of your hand. Silicon is also one of the most important metalloids of the p-block group as it is an important component of glass.
P block elements consist of:
- Group 13 Elements: Boron family
- Group 14 Elements: Carbon family
- Group 15 Elements: Nitrogen family
- Group 16 Elements: Oxygen family
- Group 17 Elements: Fluorine family
- Group 18 Elements: Neon family