The correct statement(s) about actinides is/are:
Show Hint
- The 5f electrons of actinides are bound less tightly than the 4f electrons.
- The trans uranium elements are prepared artificially.
- All the actinides are radioactive.
- Actinides do not exhibit actinide contraction.
The Correct Option is A, B, C
Solution and Explanation
Step 1: Analyze the Properties of Actinides.
- (A) The 5f electrons of actinides are more loosely bound compared to the 4f electrons of lanthanides due to the larger size of the actinides.
- (B) The trans-uranium elements (elements with atomic number greater than uranium) are indeed prepared artificially in laboratories.
- (C) All actinides are radioactive, as they have unstable nuclei.
- (D) Actinide contraction is observed, similar to lanthanide contraction, due to the poor shielding of the 5f electrons.
Step 2: Conclusion.
The correct statements are (A), (B), and (C). Therefore, the correct answers are (A), (B), and (C).
Final Answer: \[ \boxed{(A) \text{ The 5f electrons of actinides are bound less tightly than the 4f electrons} (B) \text{ The trans uranium elements are prepared artificially} (C) \text{ All the actinides are radioactive}} \]
Top Questions on Phase rule
- Let \( p(\bar{p}, \bar{q}, t) \) be the phase space density of an ensemble of a system. The Hamiltonian of the system is \( H(p, q) \). If \( \{A, B\} \) denotes the Poisson bracket of \( A \) and \( B \), then \( \frac{dp}{dt} = 0 \) implies
- GATE PH - 2024
- Electronics
- Phase rule
- The following figure shows an experimental liquid-liquid phase diagram of phenol and water at the vapor pressure of the system. The total amount of phenol and water (in mol) present in the phenol-rich phase when 5 mol of water was shaken with 5 mol of phenol at 40 °C is _______.
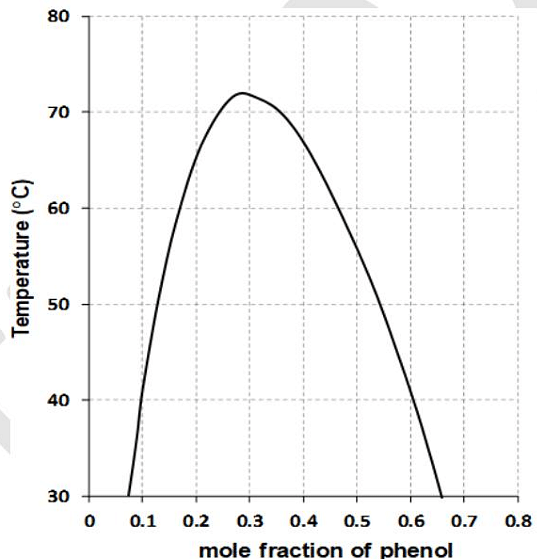
(rounded off to one decimal place) - The phase space is a
- CUET (PG) - 2023
- Physics
- Phase rule
- The Lissajous figure may be a straight line if the phase difference is
- CUET (PG) - 2023
- Physics
- Phase rule
- A closed system consists of a solution of liquid water and ethanol in equilibrium with its vapours. Using the Gibbs phase rule, the degree of freedom of the system is:
Questions Asked in GATE CY exam
- For a first-order reaction, the unit of rate constant is:
- GATE CY - 2026
- Chemical Kinetics
- Which ligand causes maximum crystal field splitting?
- GATE CY - 2026
- Coordination chemistry
- The standard electrode potential of the standard hydrogen electrode (SHE) is:
- GATE CY - 2026
- Electrochemistry
- Which of the following reagents converts an aldehyde selectively into a primary alcohol?
- GATE CY - 2026
- Organic Chemistry
- In IR spectroscopy, which bond absorbs at the highest wavenumber?
- GATE CY - 2026
- Spectroscopy