Question:
A closed system consists of a solution of liquid water and ethanol in equilibrium with its vapours. Using the Gibbs phase rule, the degree of freedom of the system is:
A closed system consists of a solution of liquid water and ethanol in equilibrium with its vapours. Using the Gibbs phase rule, the degree of freedom of the system is:
Show Hint
The Gibbs phase rule helps in determining the number of independent variables (such as pressure, temperature) that can be specified in a system at equilibrium.
Updated On: Jan 6, 2026
- 0
- 1
- 2
- 3
Hide Solution
Verified By Collegedunia
The Correct Option is C
Solution and Explanation
The Gibbs phase rule is used to determine the degree of freedom in a system. The formula for the degree of freedom (\( F \)) is given by:
\[
F = C - P + 2,
\]
where \( C \) is the number of components and \( P \) is the number of phases. In this case:
- The system consists of two components: liquid water and ethanol.
- The system is in equilibrium with its vapours, meaning there are two phases (liquid and vapour).
Thus, the degree of freedom is: \[ F = 2 - 2 + 2 = 2. \] Hence, the correct answer is \( (C) 2 \). Final Answer: (C) 2
- The system consists of two components: liquid water and ethanol.
- The system is in equilibrium with its vapours, meaning there are two phases (liquid and vapour).
Thus, the degree of freedom is: \[ F = 2 - 2 + 2 = 2. \] Hence, the correct answer is \( (C) 2 \). Final Answer: (C) 2
Was this answer helpful?
0
0
Top Questions on Phase rule
- Let \( p(\bar{p}, \bar{q}, t) \) be the phase space density of an ensemble of a system. The Hamiltonian of the system is \( H(p, q) \). If \( \{A, B\} \) denotes the Poisson bracket of \( A \) and \( B \), then \( \frac{dp}{dt} = 0 \) implies
- GATE PH - 2024
- Electronics
- Phase rule
- The following figure shows an experimental liquid-liquid phase diagram of phenol and water at the vapor pressure of the system. The total amount of phenol and water (in mol) present in the phenol-rich phase when 5 mol of water was shaken with 5 mol of phenol at 40 °C is _______.
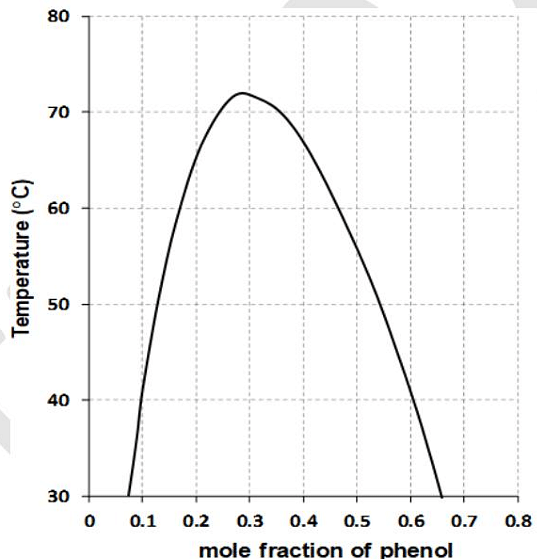
(rounded off to one decimal place) - The phase space is a
- CUET (PG) - 2023
- Physics
- Phase rule
- The Lissajous figure may be a straight line if the phase difference is
- CUET (PG) - 2023
- Physics
- Phase rule
- The correct statement(s) about actinides is/are:
Questions Asked in GATE XE exam
The value of the determinant

is:
- GATE XE - 2026
- Determinants
- The Poisson’s ratio for an incompressible material is:
- GATE XE - 2026
- Material Science
- The mode of heat transfer which does not require a material medium is:
- GATE XE - 2026
- Material Science
- Hooke’s law is valid up to:
- GATE XE - 2026
- Strength of Materials
- Which crystal structure is exhibited by body-centered cubic (BCC) metals?
- GATE XE - 2026
- Material Science
View More Questions