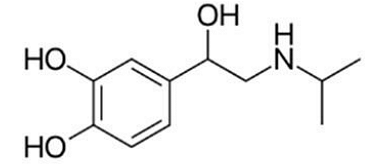
The below structure represent the drug:

Show Hint
- Catecholamines like Isoprenaline, Norepinephrine, and Dopamine have hydroxyl (-OH) groups on a benzene ring.
- Isoprenaline is unique because it contains a methyl (\(- \text{CH}_3 \)) substitution on the amine group, differentiating it from Norepinephrine.
- Isoprenaline
- Amphetamine
- Norepinephrine
- Salbutamol
The Correct Option is A
Solution and Explanation
Step 1: Identifying the Structural Features The given structure corresponds to Isoprenaline, also known as Isoproterenol. The key identifying features include: A benzene ring with two hydroxyl groups (-OH) attached at positions 1 and 2, forming a catechol structure. A side chain containing a primary amine (-NH group), a characteristic feature of sympathomimetic drugs.
Step 2: Differentiating from Similar Compounds - Isoprenaline vs. Norepinephrine: - Norepinephrine has a hydroxy (-OH) group on the side chain, whereas Isoprenaline does not.
- Isoprenaline contains an additional methyl \( \text{CH}_3 \) group attached to the amine, which distinguishes it from norepinephrine.
- Isoprenaline vs. Amphetamine: - Amphetamine lacks the catechol hydroxyl groups and instead has a simple benzene ring with an amine group.
- Isoprenaline vs. Salbutamol: - Salbutamol has a tert-butyl group on the amine instead of a methyl group and is primarily used for asthma treatment.
Step 3: Confirming the Identity The presence of the catechol hydroxyl groups and the methyl-substituted amine confirms that the given structure is Isoprenaline.
Top Questions on Biogenetic pathways
- The below structure represent the drug:

- GPAT - 2024
- Pharmacognosy
- Biogenetic pathways
- Which one is not the characteristics of the Hexose Monophosphate Pathway?
- GPAT - 2023
- Pharmacognosy
- Biogenetic pathways
- Arrange the following intermediates in the synthesis of isoprenoids in the right sequence
A. Squalence
B. Farnesyl PP
C. Geranyl PP
D. Acetyl CoA
E. Mevalonate
Choose the correct answer from the options given below:- GPAT - 2023
- Pharmacognosy
- Biogenetic pathways
- Which one of the following is an autosomal dominant syndrome in its inheritance?
- GPAT - 2023
- Pharmacognosy
- Biogenetic pathways
- Which of the following genes responsible for graft rejection in humans?
- GPAT - 2023
- Pharmacognosy
- Biogenetic pathways
Questions Asked in GPAT exam
- Match the following:
(1) Schedule FF
(2) Schedule F3
(3) Schedule V
(4) Schedule Y
Descriptions:
(P) Standards of patent and proprietary medicines
(Q) Requirements and guidelines for clinical trials
(R) Standards for sterilized umbilical tapes
(S) Standards for Ophthalmic preparations- GPAT - 2025
- Drug therapy
- If the label or the container bears the name of an individual or company purporting to be the manufacturer of the drug, which individual or company is fictitious or does not exist, it is:
- GPAT - 2025
- Drug therapy
- Manufacturing Specification for tooling has been standardized by?
- GPAT - 2025
- Pharmacy Profession & Introduction to Pharmaceuticals
- As per USP, the maximum concentration of benzalkonium chloride used as a preservative in parenteral formulations is
- GPAT - 2025
- Pharmaceutical Analysis
Match the following:
(P) Schedule H
(Q) Schedule G
(R) Schedule P
(S) Schedule F2
Descriptions:
(I) Life period of drugs
(II) Drugs used under RMP
(III) List of Prescription Drugs
(IV) Standards for surgical dressing
- GPAT - 2025
- Drug therapy