Sucrose on hydrolysis gives
- $\beta-D-Glucose$ + $\alpha -D-Fructose$
- $\alpha-D-Glucose + \beta - D - Glucose$
- $\alpha-D-Glucose + \beta -D-Fructose$
- $\alpha-D-Fructose + \beta -D-Fructose$
The Correct Option is C
Approach Solution - 1
The correct answer is Option C) \(\alpha-D-Glucose + \beta -D-Fructose\)
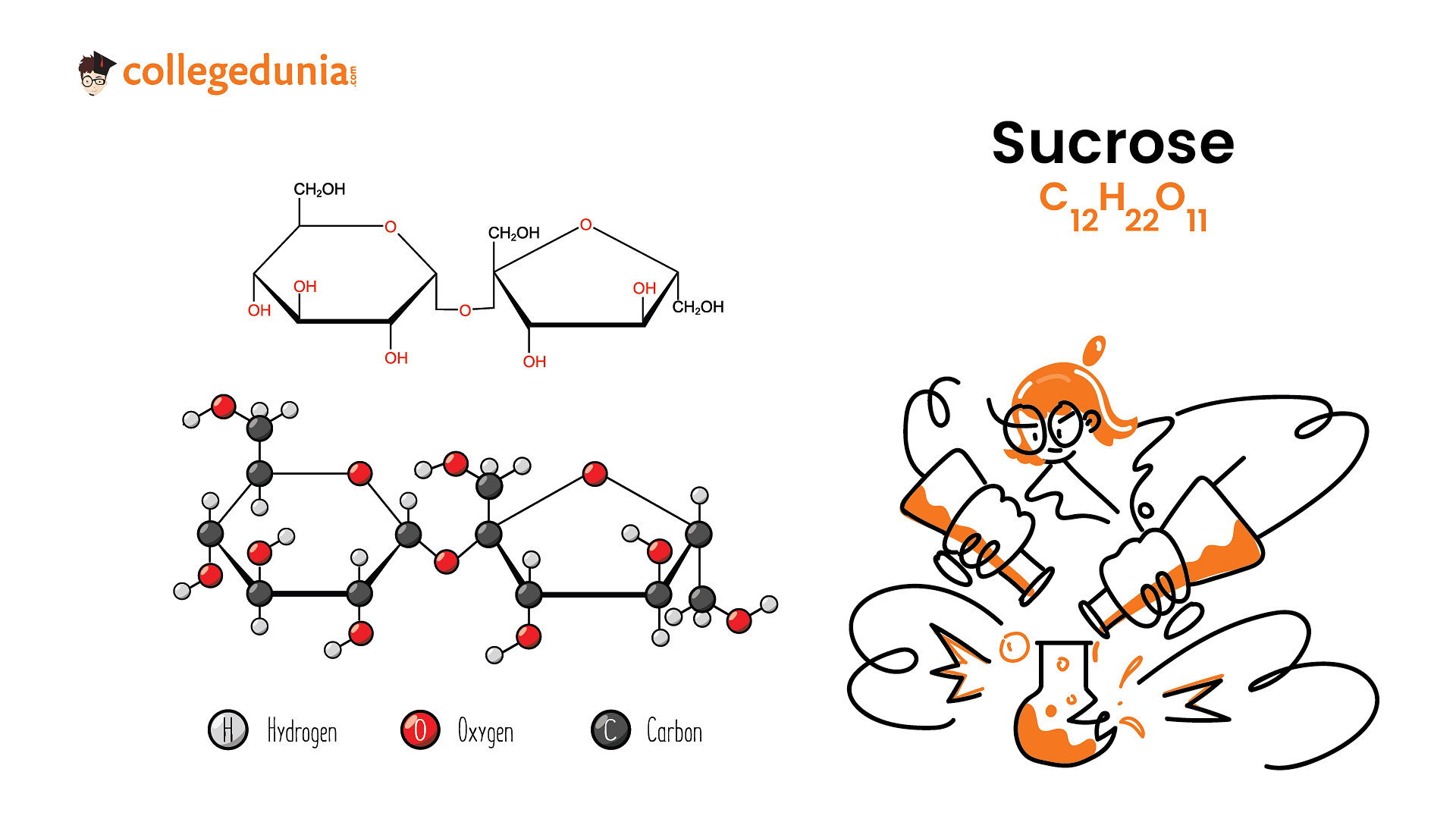
Sucrose is a disaccharide made up of two monosaccharides, namely, glucose and fructose. Sucrose is naturally produced in plants and is refined to form edible table sugar. The molecular formula of sucrose is C12H22O11.
The two monosaccharides in sucrose are held together by glycosidic linkage. Upon hydrolysis, this glycosidic linkage is broken to yield two molecules of \(\alpha\) - D - glucose and \(\beta\) - D - fructose. The reaction can be written as:
\(C_{12}H_{22}O_{11}+H_{2}O \to\underset{\text{D(+)Glucose}}{{C_{6}H_{12}O_{6}}}+\underset{\text{D(-)fructose}}{{C_{6}H_{12}O_{6}}}\)
In expanded form,

Read more from the chapter: Biomolecules
Approach Solution -2
The correct answer is Option C) \(\alpha-D-Glucose + \beta -D-Fructose\)
Real Life Applications
1. Use as Biofuel.
2. Use as sweeteners in food items.
3. Hydrolysis of sucrose produces glucose solution used in the medical industry.
4. Use as a agent to make cosmetics.
Question can also be asked as
- What enzymes are involved in the hydrolysis of sucrose?
- How does the hydrolysis of sucrose differ from its synthesis?
- What is the chemical equation for the hydrolysis of sucrose?
Approach Solution -3
The correct answer is Option C) \(\alpha-D-Glucose + \beta -D-Fructose\)
Sucrose is formed by the combination of two monosaccharides: Glucose and Fructose. The chemical formula of sucrose is C₁₂H₂₂0₁₁. It is commonly known as cane sugar or table sugar.
Properties of Sucrose
- The crystal structure of sucrose is monoclinic.
- The enthalpy of combustion for sucrose is 5647 kJ.mol⁻¹
- On combustion, sucrose forms carbon dioxide and water.
- It can be dehydrated with the help of sulphuric acid.
- There is a glycosidic bond between glucose and fructose
Read more:
Sucrose is formed by the combination of two monosaccharides: Glucose and Fructose. The chemical formula of sucrose is C₁₂H₂₂0₁₁. It is commonly known as cane sugar or table sugar.
Properties of Sucrose
- The crystal structure of sucrose is monoclinic.
- The enthalpy of combustion for sucrose is 5647 kJ.mol⁻¹
- On combustion, sucrose forms carbon dioxide and water.
- It can be dehydrated with the help of sulphuric acid.
- There is a glycosidic bond between glucose and fructose
Read more:
| Related Concepts | ||
|---|---|---|
| Carbohydrates | Glycogen | Maltose |
| Peptide bond | Amino acids | Compounds |
Top Questions on Enzymes
- Describe briefly, the charge relay system that operates in chymotrypsin enzyme.
The graph showing the concept of activation energy of enzyme is given below. Observe the graph and choose the correct option for M and N.

- Assertion (A): Restriction endonuclease recognises palindromic sequence in DNA and cuts them. Reason (R): Palindromic sequence has two unique recognition sites PstI and PvuI recognised by restriction endonuclease.
- Streptokinase produced by bacterium Streptococcus is used for:
- Which one of the following enzymes contains 'Heme' as the prosthetic group?
Questions Asked in NEET exam
- Two cities X and Y are connected by a regular bus service with a bus leaving in either direction every T min. A girl is driving scooty with a speed of 60 km/h in the direction X to Y. She notices that a bus goes past her every 30 minutes in the direction of her motion, and every 10 minutes in the opposite direction. Choose the correct option for the period T of the bus service and the speed (assumed constant) of the buses.
- NEET (UG) - 2025
- Relative Velocity
- A physical quantity P is related to four observations a, b, c, and d as follows: P = a3 b2 (c / √d) The percentage errors of measurement in a, b, c, and d are 1%, 3%, 2%, and 4% respectively. The percentage error in the quantity P is:
- NEET (UG) - 2025
- Dimensional analysis and its applications
What is Microalbuminuria ?
- NEET (UG) - 2025
- Human physiology
The output (Y) of the given logic implementation is similar to the output of an/a …………. gate.

- NEET (UG) - 2025
- Logic gates
- An oxygen cylinder of volume 30 litre has 18.20 moles of oxygen. After some oxygen is withdrawn from the cylinder, its gauge pressure drops to 11 atmospheric pressure at temperature \(27^\circ\)C. The mass of the oxygen withdrawn from the cylinder is nearly equal to: [Given, \(R = \frac{100}{12} \text{ J mol}^{-1} \text{K}^{-1}\), and molecular mass of \(O_2 = 32 \text{ g/mol}\), 1 atm pressure = \(1.01 \times 10^5 \text{ N/m}^2\)]
- NEET (UG) - 2025
- Ideal-gas equation and absolute temperature