Question:
Rancidity in the fixed oils generally show ‐
Rancidity in the fixed oils generally show ‐
Updated On: Nov 12, 2025
- Higher Iodine value as compared to the standards
- Higher Acid value as compared to the standards
- Higher Peroxide value as compared to the standards
- Higher level of Unsaponifiable matter
Hide Solution
Verified By Collegedunia
The Correct Option is B
Solution and Explanation
To understand the concept of rancidity in fixed oils and how it affects different values, we need to dive into the chemistry of oils:
- Rancidity: This is the process where oils undergo degradation due to exposure to oxygen, light, or moisture, leading to a sour taste and unpleasant odor.
- Acid Value: It measures the amount of free fatty acids present in the oil. As oils become rancid, the triglycerides can break down into free fatty acids, which raises the acid value.
Now, let's evaluate the options provided:
- Higher Iodine value as compared to the standards: The iodine value indicates the degree of unsaturation. It doesn't necessarily correlate directly with rancidity, as rancidity primarily involves oxidation, rather than the degree of unsaturation.
- Higher Acid value as compared to the standards: As explained above, rancidity results in the breakdown of triglycerides, increasing the amount of free fatty acids and, consequently, the acid value.
- Higher Peroxide value as compared to the standards: While the peroxide value measures oxidation, indicating early stages of rancidity, it's not the best measure compared to acid value, which continues to increase as rancidity progresses.
- Higher level of Unsaponifiable matter: This refers to substances in the oil that cannot form soap. It normally does not change substantially with rancidity.
Based on the reasoning above, the correct answer is that rancidity in fixed oils generally shows a Higher Acid value as compared to the standards. This is due to the accumulation of free fatty acids as the oil breaks down.
Was this answer helpful?
0
0
Top Questions on Phytopharmaceuticals
- According to the SAR of Chloroquine, electron:
- GPAT - 2024
- Pharmacognosy
- Phytopharmaceuticals
- According to the SAR of Chloroquine electron:
- GPAT - 2024
- Pharmacognosy
- Phytopharmaceuticals
- Following is not an example of carbapenem
- GPAT - 2023
- Pharmacognosy
- Phytopharmaceuticals
- Quinine and quinidine differs in:
- GPAT - 2023
- Pharmacognosy
- Phytopharmaceuticals
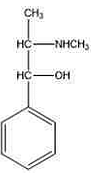
The above structure is of:- GPAT - 2022
- Pharmacognosy
- Phytopharmaceuticals
View More Questions
Questions Asked in GPAT exam
- Match the following:
(1) Schedule FF
(2) Schedule F3
(3) Schedule V
(4) Schedule Y
Descriptions:
(P) Standards of patent and proprietary medicines
(Q) Requirements and guidelines for clinical trials
(R) Standards for sterilized umbilical tapes
(S) Standards for Ophthalmic preparations- GPAT - 2025
- Drug therapy
- If the label or the container bears the name of an individual or company purporting to be the manufacturer of the drug, which individual or company is fictitious or does not exist, it is:
- GPAT - 2025
- Drug therapy
- Manufacturing Specification for tooling has been standardized by?
- GPAT - 2025
- Pharmacy Profession & Introduction to Pharmaceuticals
- As per USP, the maximum concentration of benzalkonium chloride used as a preservative in parenteral formulations is
- GPAT - 2025
- Pharmaceutical Analysis
Match the following:
(P) Schedule H
(Q) Schedule G
(R) Schedule P
(S) Schedule F2
Descriptions:
(I) Life period of drugs
(II) Drugs used under RMP
(III) List of Prescription Drugs
(IV) Standards for surgical dressing
- GPAT - 2025
- Drug therapy
View More Questions