Osmotic pressure can be increased by
Show Hint
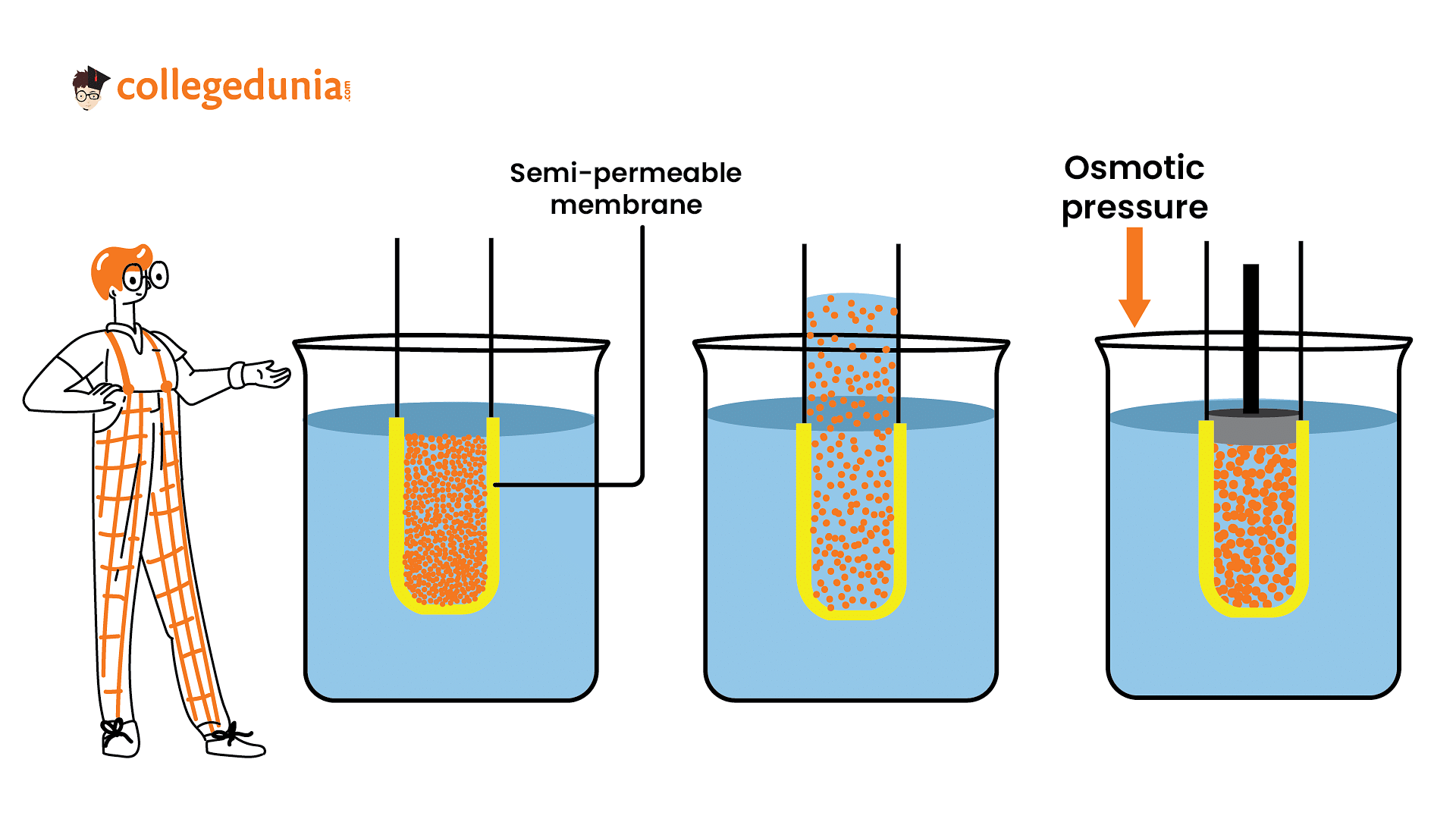
Osmotic pressure describes the pressure exerted by a solvent to prevent the influx of solvent molecules through a semipermeable membrane
- increasing the temperature of the solution.
- decreasing the temperature of the solution.
- increasing the volume of the vessel.
- diluting the solution.
The Correct Option is A
Approach Solution - 1
Osmotic pressure is directly proportional to the temperature $\because \pi=CRT$ where,
- C= concentration
- R= gas constant
- T= temperature
i.e. $\pi \propto T$, thus, on increasing the temperature, osmotic pressure also increases.
Discover More From Chapter: Solutions
Approach Solution -2
Osmotic pressure describes the pressure exerted by a solvent to prevent the influx of solvent molecules through a semipermeable membrane. The temperature of a solution has a significant influence on its osmotic pressure.
Temperature and Molecular Kinetics
- Temperature is a measure of the average kinetic energy of molecules in a solution.
- An increase in temperature corresponds to an increase in molecular kinetic energy.
Effect of Temperature on Solvent-Solute Interactions
- When the temperature of a solution increases, the kinetic energy of solvent and solute molecules increases as well.
- This increased kinetic energy leads to more frequent and energetic collisions between solvent and solute particles.
Read More:
| Related Concepts | ||
|---|---|---|
| Spore Formation | Vegetative Bud | Electroosmosis |
| Amoeba | Binomial Nomenclature | Cell Wall and Cell Membrane |
Enhanced Solvent-Solute Interaction
- As the temperature rises, solvent molecules gain energy to overcome intermolecular forces.
- This enhanced interaction increases the rate of solvent molecules crossing the semipermeable membrane.
Increased Movement of Solvent
- With higher temperatures, solvent molecules move more rapidly, resulting in a greater tendency to move across the membrane.
- Consequently, the osmotic pressure of the solution increases due to the greater flow of solvent molecules.
Relationship Between Temperature and Osmotic Pressure
- Increasing the temperature of a solution increases the kinetic energy and movement of solvent molecules.
- This increased movement elevates the osmotic pressure, as more solvent molecules attempt to equalize the concentration on both sides of the semipermeable membrane.
The temperature of a solution plays a crucial role in its osmotic pressure. Increasing the temperature leads to enhanced molecular movement and more vigorous solvent-solute interactions, resulting in an increase in osmotic pressure.
Top Questions on Solutions
- Consider the dissociation equilibrium of the following weak acid: \[ \mathrm{HA \rightleftharpoons H^+(aq) + A^-(aq)} \] If the \(pK_a\) of the acid is \(4\), then the pH of a \(10\ \text{mM}\) HA solution is ________ (Nearest integer). (Given: The degree of dissociation can be neglected with respect to unity)
- The crystal field splitting energy of $[Co(oxalate)_3]^3-$ complex is 'n' times that of the $[Cr(oxalate)_3]^3-$ complex. Here 'n' is_______ (Assume $\Delta_0 > P$)}
- The osmotic pressure of a living cell is 12 atm at 300 K. The strength of sodium chloride solution that is isotonic with the living cell at this temperature is ____________ g L$^{-1}$. (Nearest integer)
Given: R = 0.08 L atm K$^{-1}$ mol$^{-1}$
Assume complete dissociation of NaCl
(Given : Molar mass of Na and Cl are 23 and 35.5 g mol$^{-1}$ respectively.) A substance 'X' (1.5 g) dissolved in 150 g of a solvent 'Y' (molar mass = 300 g mol$^{-1}$) led to an elevation of the boiling point by 0.5 K. The relative lowering in the vapour pressure of the solvent 'Y' is $____________ \(\times 10^{-2}\). (nearest integer)
[Given : $K_{b}$ of the solvent = 5.0 K kg mol$^{-1}$]
Assume the solution to be dilute and no association or dissociation of X takes place in solution.- At \(T\) K, \(100\,\text{g}\) of \(98%\) \(H_2SO_4\) (w/w) aqueous solution is mixed with \(100\,\text{g}\) of \(49%\) \(H_2SO_4\) (w/w) aqueous solution. What is the mole fraction of \(H_2SO_4\) in the resultant solution? (Given: Atomic mass \(H = 1\,u,\; S = 32\,u,\; O = 16\,u\). Assume that temperature after mixing remains constant.)
Questions Asked in KCET exam
Match the following:
In the following, \( [x] \) denotes the greatest integer less than or equal to \( x \).
Choose the correct answer from the options given below:- KCET - 2025
- Differentiability
- If \[ y = \frac{\cos x}{1 + \sin x} \] then:
- KCET - 2025
- Differentiability
- A function \( f(x) \) is given by:
\[ f(x) = \begin{cases} \frac{1}{e^x - 1}, & \text{if } x \neq 0 \\ \frac{1}{e^x + 1}, & \text{if } x = 0 \end{cases} \] Then, which of the following is true?- KCET - 2025
- Limits
- The function f(x) is given by:
For x < 0:
f(x) = ex + axFor x ≥ 0:
f(x) = b(x - 1)2
The function is differentiable at x = 0. Then,- KCET - 2025
- Differentiability
- The function \( f(x) = \tan x - x \)
- KCET - 2025
- Derivatives
Concepts Used:
Solutions
A solution is a homogeneous mixture of two or more components in which the particle size is smaller than 1 nm.
For example, salt and sugar is a good illustration of a solution. A solution can be categorized into several components.
Types of Solutions:
The solutions can be classified into three types:
- Solid Solutions - In these solutions, the solvent is in a Solid-state.
- Liquid Solutions- In these solutions, the solvent is in a Liquid state.
- Gaseous Solutions - In these solutions, the solvent is in a Gaseous state.
On the basis of the amount of solute dissolved in a solvent, solutions are divided into the following types:
- Unsaturated Solution- A solution in which more solute can be dissolved without raising the temperature of the solution is known as an unsaturated solution.
- Saturated Solution- A solution in which no solute can be dissolved after reaching a certain amount of temperature is known as an unsaturated saturated solution.
- Supersaturated Solution- A solution that contains more solute than the maximum amount at a certain temperature is known as a supersaturated solution.