Methyl benzoate can be prepared by
- $C _{6} H _{5} COOH + CH _{3} OH \xrightarrow{ H ^{+}}$
- $C _{6} H _{5} COOCl + CH _{3} OH \xrightarrow{\text { Pyridine }}$
- $C _{6} H _{5} COOH + CH _{2} N _{2} \rightarrow$
- All of the above method
The Correct Option is D
Approach Solution - 1
Approach Solution -2
Ans. Methyl benzoate is created when benzoic acid and methanol condense in the presence of a strong acid.
- Methyl benzoate is an organic compound, an ester with the chemical formula C6H5CO2CH3.
- When dissolved in a liquid, methyl benzoate can take the form of a solid or a crystalline solid. than water, but denser.
- Contact may cause mild irritation to mucous membranes, eyes, and skin.
- By consumption, it could be somewhat hazardous. used to produce different compounds.
- Methyl benzoate, a benzoate ester, is created when methanol and benzoic acid are combined.
- It serves as an insect attractant and metabolite.
- It is both a methyl ester and a benzoate ester.
- In the Fischer Esterification reaction mechanism, an ester is formed by the conversion of carboxylic acid in the presence of excess alcohol and a strong acid as catalysts.
- The reaction was discovered by Emil Fischer.
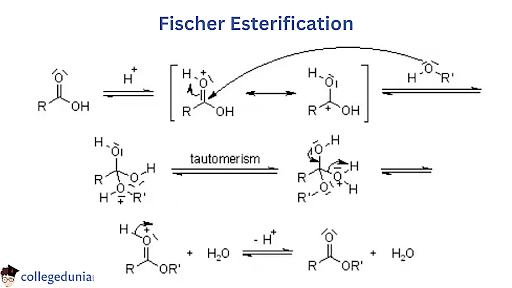
- Some disadvantages of the Fischer esterification reaction are:
- The thermodynamic reversibility of the reaction.
- The reaction rates are quite slow.
- An energy source is usually needed to promote the Fischer esterification reaction.
- This energy is usually supplied in the form of heat.
Learn with videos:
Top Questions on Aldehydes, Ketones and Carboxylic Acids
- Iodoform test can differentiate :
- JEE Main - 2026
- Chemistry
- Aldehydes, Ketones and Carboxylic Acids
Convert Ethanal to But-2-enal
- CBSE CLASS XII - 2025
- Chemistry
- Aldehydes, Ketones and Carboxylic Acids
- Write the reaction involved in the following :
Clemmensen reduction- CBSE CLASS XII - 2025
- Chemistry
- Aldehydes, Ketones and Carboxylic Acids
- An organic compound (X) having molecular formula C$_5$H$_{10$O can show various properties depending on its structures. Draw each of the structures if it :
shows Cannizzaro reaction.- CBSE CLASS XII - 2025
- Chemistry
- Aldehydes, Ketones and Carboxylic Acids
Write structure of the products of the following reactions:

- CBSE CLASS XII - 2025
- Chemistry
- Aldehydes, Ketones and Carboxylic Acids
Questions Asked in AMUEEE exam
- The ratio of the half-life time $ (t_{1/2}) $ , to the three quarter life-time, $ (t_{3/4}) $ , for a reaction that is second order
- AMUEEE - 2018
- Order of Reaction
- $ 0.002\, M $ solution of a weak acid has an equivalent conductance $ (\wedge) 60\, ohm^{-1}\, cm^{2}\, eq^{-1} $ . What will be the $ pH $ ? (Given : $ \wedge = 400 \, ohm^{-1} \, cm^{2} \, eq^{-1} $ ]
- AMUEEE - 2018
- Electrochemistry
- The electrode potential, $ E^{\circ} $ , for the reduction of $ MnO^{-}_{4} $ to $ Mn^{2+} $ in acidic medium is $ +1.51\, V $ . Which of the following metal(s) will be oxidised? The reduction reactions and standard electrode potentials for $ Zn^{2+}, Ag^{+} $ , and $ Au^{+} $ are given as $ Zn^{2+} (aq) +2e \to Zn(s), E^{\circ} = -0.762\, V $ $ Ag+ (aq) +e {\rightleftharpoons} Ag (s), E^{\circ}=+0.80 \,V $ $ Au^{+} (aq) +e {\rightleftharpoons} Au(s), E^{\circ}+1.69\,V $
- AMUEEE - 2018
- Electrochemistry
- Three particles, two with masses $ m $ and one with mass $ M $ , might be arranged in any of the four configurations shown below. Rank the configurations according to the magnitude of the gravitational force on $ M $ , least to greatest

- AMUEEE - 2018
- Gravitation
- The $ EAN $ value $ y \left[Ti\left(\sigma-C_{6}H_{5}\right)_{2}\left(\pi-C_{5}H_{5}\right)_{2}\right]^{0} $ is
- AMUEEE - 2018
- coordination compounds
Concepts Used:
Aldehydes, Ketones, and Carboxylic Acids
Aldehydes, Ketones, and Carboxylic Acids are carbonyl compounds that contain a carbon-oxygen double bond. These organic compounds are very important in the field of organic chemistry and also have many industrial applications.
Aldehydes:
Aldehydes are organic compounds that have the functional group -CHO.
Preparation of Aldehydes
Acid chlorides are reduced to aldehydes with hydrogen in the presence of palladium catalyst spread on barium sulfate.
Ketones:
Ketones are organic compounds that have the functional group C=O and the structure R-(C=O)-R’.
Preparation of Ketones
Acid chlorides on reaction with dialkyl cadmium produce ketones. Dialkyl cadmium themselves are prepared from Grignard reagents.
Carboxylic Acid:
Carboxylic acids are organic compounds that contain a (C=O)OH group attached to an R group (where R refers to the remaining part of the molecule).
Preparation of Carboxylic Acids
Primary alcohols are readily oxidized to carboxylic acids with common oxidizing agents such as potassium permanganate in neutral acidic or alkaline media or by potassium dichromate and chromium trioxide in acidic media.
