Major product formed in the given reaction is
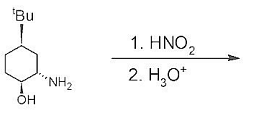
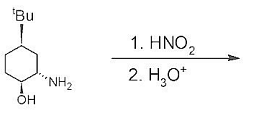
The Correct Option is B
Solution and Explanation
To determine the major product formed in the given reaction, we need to look at the reagents and reaction conditions involved:
- The reaction starts with cyclohexylamine with a tertiary butyl group and a hydroxyl group on the adjacent carbon.
- The first reagent, \(HNO_2\) (nitrous acid), is known for converting primary amines into diazonium salts, which are unstable when aliphatic and decompose to form alcohols, alkenes, or other products depending on the conditions.
- In this case, since the compound is not aromatic and has steric hindrance due to the tertiary butyl group, the diazonium salt formed will quickly lose nitrogen gas (a very stable leaving group), resulting in a carbocation intermediate.
- The presence of the adjacent hydroxyl group allows for rearrangement or immediate substitution, leading to intramolecular formation of a more stable compound.
- The second step with \(H_3O^+\) (acidic conditions) will promote reactions like elimination or further stabilization of any carbocation intermediates.
- Taking into account these possibilities, the most stable product formed would be the one where the tertiary butyl group and the alcohol group stabilize the intermediate. This logic supports the formation of an internal ether or any rearranged cyclic compound.
By examining the options and considering potential rearrangements, we identify the second option as the major product:

This cyclic structure is stable given the conditions and reagents present in the reaction. Consequently, Option 2 is the correct choice.
Top Questions on Chemical Reaction
- In a batch reactor, the reaction rate is:
- TS PGECET - 2025
- Chemical Engineering
- Chemical Reaction
- Sequence of Reactions of Ethylene and Chlorine to form PVC:
- GATE CH - 2025
- Chemical Engineering
- Chemical Reaction
- Mean Residence Time Calculation for a given system: \[ t_m = \int_0^{\infty} t E(t) \, dt \] Where \( E(t) \) is the distribution function. For the given system, the distribution function is: \[ E(t) = 1 - 2t, \quad t \leq 0.5 \] \[ E(t) = 0, \quad t > 0.5 \]
- GATE CH - 2025
- Chemical Engineering
- Chemical Reaction
- What is the first stage of production of paper from wood?
- GATE CH - 2025
- Chemical Engineering
- Chemical Reaction
\(\text{Reaction of aniline with conc. HNO}_3 \text{ and conc. H}_2\text{SO}_4 \text{ at 298 K will produce 47\% of:}\)
- CUET (UG) - 2024
- Chemistry
- Chemical Reaction
Questions Asked in IIT JAM BT exam
- The wavelength of a photon emitted during a transition from \( n = 3 \) to \( n = 2 \) state in the H atom is .............. nm. (answer in integer).
- IIT JAM BT - 2025
- Biotechnology
- The porphyrin ring (tetrapyrrole structure) is NOT found in functional
- IIT JAM BT - 2025
- Biotechnology
Identify the taxa that constitute a paraphyletic group in the given phylogenetic tree.

- IIT JAM BT - 2025
- Genetics
The vector, shown in the figure, has promoter and RBS sequences in the 300 bp region between the restriction sites for enzymes X and Y. There are no other sites for X and Y in the vector. The promoter is directed towards the Y site. The insert containing only an ORF provides 3 fragments after digestion with both enzymes X and Y. The ORF is cloned in the correct orientation in the vector using the single restriction enzyme Y. The size of the largest fragment of the recombinant plasmid expressing the ORF upon digestion with enzyme X is ........... bp. (answer in integer)

- IIT JAM BT - 2025
- Biotechnology
- Match the animals in Group I with the major form of excreted nitrogen metabolite in Group II. \[ \begin{array}{c|c} \text{Group I} & \text{Group II} \\ \hline P:\; \text{Bony fishes} & 3:\; \text{Ammonia} \\ Q:\; \text{Lions} & 1:\; \text{Urea} \\ R:\; \text{Birds} & 2:\; \text{Uric acid} \\ \end{array} \]
- IIT JAM BT - 2025
- Metabolism


