Question:
Identify the correct reagents that would bring about the following transformation.
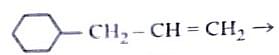
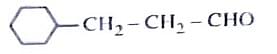
Identify the correct reagents that would bring about the following transformation.
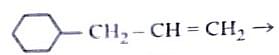
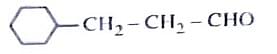
Updated On: Jan 13, 2026
- (i) H2O/H+
(ii) CrO3 - (i) BH3
(ii) \(H_2O_2/O^\ominus H\)
(iii) PCC - (i) BH3
(ii) \(H_2O_2/O^\ominus H\)
(iii) alk. KMnO4 - (iv)\(H_3O^\oplus\)
(i) H2O/H+
(ii) PCC
Hide Solution
Verified By Collegedunia
The Correct Option is B
Solution and Explanation
Step 1: Understanding the Given Transformation
The transformation likely involves oxidation or hydroboration-oxidation, depending on the functional groups present.
Step 2: Analyze the Reagents
- \( BH_3 \) followed by \( H_2O_2/OH^- \): This is the hydroboration-oxidation reaction, which converts an alkene to an anti-Markovnikov alcohol.
- PCC (Pyridinium Chlorochromate): A mild oxidizing agent that converts primary alcohols to aldehydes and secondary alcohols to ketones.
- Chromic acid (\( CrO_3 \)) in acidic medium: A strong oxidizing agent that converts alcohols to carboxylic acids.
- Acidic water (\( H_2O/H^+ \)): Used for hydration of alkenes to form Markovnikov alcohols.
Step 3: Identify the Correct Set of Reagents
Since the transformation involves hydration followed by oxidation, the correct sequence of reagents is:
- \( BH_3 \) (Hydroboration step).
- \( H_2O_2/OH^- \) (Oxidation to form alcohol).
- \( PCC \) (Mild oxidation to aldehyde).
Step 4: Conclusion
The correct answer is:
Option (2): (i) \( BH_3 \), (ii) \( H_2O_2/OH^- \), (iii) PCC.
Was this answer helpful?
4
2
Top Questions on Chemical Reactions
- One mole of Cl$_2$(g) was passed into 2 L of cold 2 M KOH solution. After the reaction, the concentrations of Cl$^-$, ClO$^-$ and OH$^-$ are respectively (assume volume remains constant)
- JEE Main - 2026
- Chemistry
- Chemical Reactions
The colour of the solution observed after about 1 hour of placing iron nails in copper sulphate solution is:
- CBSE Class X - 2025
- Science
- Chemical Reactions
- Example of thermal decomposition reaction are:
- \( \text{2AgCl} \rightarrow \text{2Ag} + \text{Cl}_2 \)
- \( \text{CaCO}_3 \rightarrow \text{CaO} + \text{CO}_2 \)
- \( \text{2H}_2\text{O} \rightarrow \text{2H}_2 + \text{O}_2 \)
- \( \text{2KClO}_3 \rightarrow \text{2KCl} + \text{3O}_2 \)
- CBSE Class X - 2025
- Science
- Chemical Reactions
- In which one of the following situations a chemical reaction does not occur?
- CBSE Class X - 2025
- Science
- Chemical Reactions
- The colour of the solution observed after about 1 hour of placing iron nails in copper sulphate solution is:
- CBSE Class X - 2025
- Science
- Chemical Reactions
View More Questions
Questions Asked in NEET exam
- Two cities X and Y are connected by a regular bus service with a bus leaving in either direction every T min. A girl is driving scooty with a speed of 60 km/h in the direction X to Y. She notices that a bus goes past her every 30 minutes in the direction of her motion, and every 10 minutes in the opposite direction. Choose the correct option for the period T of the bus service and the speed (assumed constant) of the buses.
- NEET (UG) - 2025
- Relative Velocity
- A physical quantity P is related to four observations a, b, c, and d as follows: P = a3 b2 (c / √d) The percentage errors of measurement in a, b, c, and d are 1%, 3%, 2%, and 4% respectively. The percentage error in the quantity P is:
- NEET (UG) - 2025
- Dimensional analysis and its applications
What is Microalbuminuria ?
- NEET (UG) - 2025
- Human physiology
The output (Y) of the given logic implementation is similar to the output of an/a …………. gate.

- NEET (UG) - 2025
- Logic gates
- An oxygen cylinder of volume 30 litre has 18.20 moles of oxygen. After some oxygen is withdrawn from the cylinder, its gauge pressure drops to 11 atmospheric pressure at temperature \(27^\circ\)C. The mass of the oxygen withdrawn from the cylinder is nearly equal to: [Given, \(R = \frac{100}{12} \text{ J mol}^{-1} \text{K}^{-1}\), and molecular mass of \(O_2 = 32 \text{ g/mol}\), 1 atm pressure = \(1.01 \times 10^5 \text{ N/m}^2\)]
- NEET (UG) - 2025
- Ideal-gas equation and absolute temperature
View More Questions