For a double strand DNA, one strand is given below:
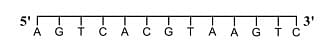
The amount of energy required to split the double strand DNA into two single strands is _____ kcal mol−1.
[Given: Average energy per H-bond for A-T base pair = 1.0 kcal mol−1, G-C base pair = 1.5 kcal mol−1, and A-U base pair = 1.25 kcal mol−1. Ignore electrostatic repulsion between the phosphate groups.]

The amount of energy required to split the double strand DNA into two single strands is _____ kcal mol−1.
[Given: Average energy per H-bond for A-T base pair = 1.0 kcal mol−1, G-C base pair = 1.5 kcal mol−1, and A-U base pair = 1.25 kcal mol−1. Ignore electrostatic repulsion between the phosphate groups.]
Correct Answer: 41
Approach Solution - 1
Calculation of Energy Required for DNA Denaturation
The given DNA sequence contains:
- Seven adenine-thymine (A-T) base pairs.
- Six guanine-cytosine (G-C) base pairs.
Step 1: Energy Contributions
Each A-T pair contributes 2 hydrogen bonds, each requiring 1 kcal of energy:
\[ 7 \times 2 \times 1 = 14 \, \text{kcal} \]
Each G-C pair contributes 3 hydrogen bonds, each requiring 1.5 kcal of energy:
\[ 6 \times 3 \times 1.5 = 27 \, \text{kcal} \]
Step 2: Total Energy Required
\[ \text{Total Energy} = 14 + 27 = 41 \, \text{kcal} \]
Final Answer:
The total energy required for DNA denaturation is 41 kcal.
Approach Solution -2
To solve the problem, we need to calculate the total energy required to break all hydrogen bonds in the given double-stranded DNA sequence.
1. Given DNA strand:
5’ — A G T C A C G T A A G T C — 3’
2. Complementary strand:
Using base pairing rules:
A pairs with T, G with C, T with A, C with G, etc.
Complementary strand (3’ to 5’):
3’ — T C A G T G C A T T C A G — 5’
3. Count number of A-T and G-C base pairs:
Count each base pair along the strand:
- A-T pairs: Count bases A in given strand (positions 1, 5, 9, 10)
Number of A-T pairs = 4
- G-C pairs: Count bases G and C in given strand:
G at positions 2, 7, 11 (3 times)
C at positions 4, 6, 12 (3 times)
Total G-C pairs = 6
4. Calculate energy contributed by each base pair:
- Energy per A-T pair = 2 H-bonds × 1.0 kcal/mol = 2.0 kcal/mol
- Energy per G-C pair = 3 H-bonds × 1.5 kcal/mol = 4.5 kcal/mol
5. Calculate total energy required to split DNA strands:
\[ E = (\text{number of A-T pairs} \times 2.0) + (\text{number of G-C pairs} \times 4.5) \] \[ E = (4 \times 2.0) + (6 \times 4.5) = 8 + 27 = 35 \, \text{kcal/mol} \]
6. Check for missing pairs:
The strand has 13 bases, meaning 12 base pairs.
We counted 4 A-T and 6 G-C pairs = 10 pairs total.
Remaining 2 bases at positions 8 and 13:
- Position 8 = T (pairs with A)
- Position 13 = C (pairs with G)
Add these pairs:
- T-A pair (1 more A-T pair)
- C-G pair (1 more G-C pair)
7. Update counts and energy:
- A-T pairs = 4 + 1 = 5
- G-C pairs = 6 + 1 = 7
Total energy:
\[ E = (5 \times 2.0) + (7 \times 4.5) = 10 + 31.5 = 41.5 \, \text{kcal/mol} \]
Final Answer:
The energy required to split the double strand DNA into two single strands is approximately \(\boxed{41}\) kcal mol\(^{-1}\).
Top Questions on Biomolecules
In the given pentapeptide, find out an essential amino acid (\(Y\)) and the sequence present in the pentapeptide.

Choose the correct answer from the options given below:- JEE Main - 2026
- Chemistry
- Biomolecules
Given below are two statements for the following reaction sequence:

- JEE Main - 2026
- Chemistry
- Biomolecules
- The correct trend in the first ionization enthalpies of the elements in the 3rd period of periodic table is :
- JEE Main - 2026
- Chemistry
- Biomolecules
Match List-I with List-II.

- JEE Main - 2026
- Chemistry
- Biomolecules
- The correct statements are :
A. Activation energy for enzyme catalysed hydrolysis of sucrose is lower than that of acid catalysed hydrolysis.
B. During denaturation, secondary and tertiary structures of a protein are destroyed but primary structure remains intact.
C. Nucleotides are joined together by glycosidic linkage between \( C_1 \) and \( C_4 \) carbons of the pentose sugar.
D. Quaternary structure of proteins represents overall folding of the polypeptide chain.
Choose the correct answer from the options given below :- JEE Main - 2026
- Chemistry
- Biomolecules
Questions Asked in JEE Advanced exam
- Let $ x_0 $ be the real number such that $ e^{x_0} + x_0 = 0 $. For a given real number $ \alpha $, define $$ g(x) = \frac{3xe^x + 3x - \alpha e^x - \alpha x}{3(e^x + 1)} $$ for all real numbers $ x $. Then which one of the following statements is TRUE?
- JEE Advanced - 2025
- Fundamental Theorem of Calculus
Let $ P(x_1, y_1) $ and $ Q(x_2, y_2) $ be two distinct points on the ellipse $$ \frac{x^2}{9} + \frac{y^2}{4} = 1 $$ such that $ y_1 > 0 $, and $ y_2 > 0 $. Let $ C $ denote the circle $ x^2 + y^2 = 9 $, and $ M $ be the point $ (3, 0) $. Suppose the line $ x = x_1 $ intersects $ C $ at $ R $, and the line $ x = x_2 $ intersects $ C $ at $ S $, such that the $ y $-coordinates of $ R $ and $ S $ are positive. Let $ \angle ROM = \frac{\pi}{6} $ and $ \angle SOM = \frac{\pi}{3} $, where $ O $ denotes the origin $ (0, 0) $. Let $ |XY| $ denote the length of the line segment $ XY $. Then which of the following statements is (are) TRUE?
- JEE Advanced - 2025
- Conic sections
Monocyclic compounds $ P, Q, R $ and $ S $ are the major products formed in the reaction sequences given below.
The product having the highest number of unsaturated carbon atom(s) is:
- JEE Advanced - 2025
- Organic Chemistry
- Adsorption of phenol from its aqueous solution on to fly ash obeys Freundlich isotherm. At a given temperature, from 10 mg g$^{-1}$ and 16 mg g$^{-1}$ aqueous phenol solutions, the concentrations of adsorbed phenol are measured to be 4 mg g$^{-1}$ and 10 mg g$^{-1}$, respectively. At this temperature, the concentration (in mg g$^{-1}$) of adsorbed phenol from 20 mg g$^{-1}$ aqueous solution of phenol will be ____. Use: $\log_{10} 2 = 0.3$
- JEE Advanced - 2025
- Adsorption
- At 300 K, an ideal dilute solution of a macromolecule exerts osmotic pressure that is expressed in terms of the height (h) of the solution (density = 1.00 g cm$^{-3}$) where h is equal to 2.00 cm. If the concentration of the dilute solution of the macromolecule is 2.00 g dm$^{-3}$, the molar mass of the macromolecule is calculated to be $X \times 10^{4}$ g mol$^{-1}$. The value of $X$ is ____. Use: Universal gas constant (R) = 8.3 J K$^{-1}$ mol$^{-1}$ and acceleration due to gravity (g) = 10 m s$^{-2}\}$
- JEE Advanced - 2025
- Colligative Properties