Question:
: At high pressure, the compression factor $Z$ is $\left(1+\frac{P b}{R T}\right)$.
: At high pressure van der Waals' equation is modified as $P(V-b)=R T$.
: At high pressure, the compression factor $Z$ is $\left(1+\frac{P b}{R T}\right)$.
: At high pressure van der Waals' equation is modified as $P(V-b)=R T$.
Updated On: Jul 28, 2022
- If both Assertion and Reason are true and Reason is the correct explanation of Assertion.
- If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
- If Assertion is true but Reason is false.
- If both Assertion and Reason are false.
Hide Solution
Verified By Collegedunia
The Correct Option is A
Solution and Explanation
van der Waals' equation is
$\left(P+\frac{a}{V^{2}}\right)(V-b)=R T$
at high pressure
$P(V-b)=R T$
$P V-P b=R T$
$\frac{P V}{R T}=\left(1+\frac{P b}{R T}\right)$
if $\frac{P V}{R T}=Z,$ then
$Z=\left(1+\frac{P b}{R T}\right)$
Was this answer helpful?
0
0
Top Questions on Van Der Waals equation
- Molar volume ($ V_m $) of a van der Waals gas can be calculated by expressing the van der Waals equation as a cubic equation with $ V_m $ as the variable. The ratio (in mol dm$^{-3}$) of the coefficient of $ V_m^2 $ to the coefficient of $ V_m $ for a gas having van der Waals constants $ a = 6.0 \, \text{dm}^6 \, \text{atm mol}^{-2} $ and $ b = 0.060 \, \text{dm}^3 \, \text{mol}^{-1} $ at 300 K and 300 atm is ____. Use: Universal gas constant $ R = 0.082 \, \text{dm}^3 \, \text{atm mol}^{-1} \, \text{K}^{-1} $
- JEE Advanced - 2025
- Chemistry
- Van Der Waals equation
- Arrange the following gases in increasing order of van der Waals constant 'a'
A. Ar
B. CH4
C. H₂O
D. C6H6
Choose the correct option from the following.- JEE Main - 2023
- Chemistry
- Van Der Waals equation
- Which of the following statemnt is incorect for physisorption?
- GUJCET - 2023
- Chemistry
- Van Der Waals equation
- At low pressure, the van der Waal's equation is reduced to
- VITEEE - 2019
- Chemistry
- Van Der Waals equation
- If $V$ is the volume of one molecule of gas under given conditions, the van der Waal?s constant $b$ is
- BITSAT - 2018
- Chemistry
- Van Der Waals equation
View More Questions
Questions Asked in AIIMS exam
- The element Neodymium (Nd) belongs to the 4f series. What is its atomic number?
- AIIMS - 2024
- Modern Periodic Law And The Present Form Of The Periodic Table
- The correct increasing order of energy of orbitals in a hydrogen atom is:
- AIIMS - 2024
- Atomic Structure
- Which of the following is a globular protein?
- AIIMS - 2024
- Biomolecules
- Given that the surface charge density on a sphere is 200 μC/m2, what is the electric field at the surface of the sphere?
- AIIMS - 2024
- Electrostatics
- Which of the following is a crystalline solid?
- AIIMS - 2024
- The solid state
View More Questions
Concepts Used:
Van Der Waals Equation
Van der Waals equation is an equation relating the relationship between the pressure, volume, temperature, and amount of real gases.
Read More: Derivation of Van Der Waals Equation
Derivation of Van der Waals equation:
For a real gas containing ‘n’ moles, the equation is written as
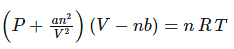
Where, P, V, T, n are the pressure, volume, temperature and moles of the gas. ‘a’ and ‘b’ constants specific to each gas.
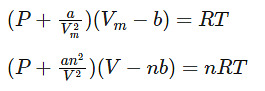
Where,
Vm: molar volume of the gas
R: universal gas constant
T: temperature
P: pressure
V: volume
Thus, Van der Waals equation can be reduced to ideal gas law as PVm = RT.
The equation can further be written as;
- Cube power of volume:
- Reduced equation (Law of corresponding states) in terms of critical constants:
Units of Van der Waals equation Constants
a: atm lit² mol-²
b: litre mol-¹