Question:
An epoxy ring can be prepared by
An epoxy ring can be prepared by
Updated On: Nov 13, 2025
- DCC oxidation of alcohol
- Prevost reaction
- Darzens glycidic ester synthesis
- Swern oxidation
Hide Solution
Verified By Collegedunia
The Correct Option is C
Solution and Explanation
The question asks for a method to prepare an epoxy ring. Let's evaluate the options given:
- DCC Oxidation of Alcohol: DCC (Dicyclohexylcarbodiimide) is commonly used for forming amides and not for oxidation. It is not relevant to forming epoxy rings.
- Prevost Reaction: This reaction involves the transformation of alkenes to glycols (vicinal diols) and some related modifications, but it does not directly lead to the formation of epoxy rings.
- Darzens Glycidic Ester Synthesis: This is a well-known method for synthetizing epoxy rings. In this reaction, an α-halo ketone reacts with a base to form an α,β-epoxy ester. This is the correct method for preparing an epoxy ring.
- Swern Oxidation: This method is used for converting primary and secondary alcohols to aldehydes and ketones, respectively. It does not facilitate epoxy ring formation.
Based on the explanations above, the correct answer is Darzens glycidic ester synthesis, as it directly involves the preparation of epoxides.
The general form of this reaction involves the nucleophilic attack of an α-halo ketone by a base, followed by cyclization to form the epoxy ring. This method is not only fundamental for synthesizing epoxy rings but also for various synthetic applications in organic chemistry.
Was this answer helpful?
0
0
Top Questions on Polycyclic aromatic hydrocarbons
- Which of the following compounds will have the highest order of their reactivity towards electrophilic substitution reaction?

Choose the correct answer from the options given below:- CUET (PG) - 2024
- Chemistry
- Polycyclic aromatic hydrocarbons
- Which of the following molecules is aromatic?

- CUET (PG) - 2024
- Chemistry
- Polycyclic aromatic hydrocarbons
- Identify the product in the following chemical reaction:
- CUET (PG) - 2024
- Chemistry
- Polycyclic aromatic hydrocarbons
- Which of the following isomer of 1, 2-cyclohexanedicarboxylic acid forms an hydride on heating?

Choose the correct answer from the options given below:- CUET (PG) - 2024
- Chemistry
- Polycyclic aromatic hydrocarbons
- The major product formed in the above reaction is:
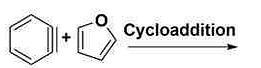
- GPAT - 2022
- Organic Chemistry
- Polycyclic aromatic hydrocarbons
View More Questions
Questions Asked in GPAT exam
- Match the following:
(1) Schedule FF
(2) Schedule F3
(3) Schedule V
(4) Schedule Y
Descriptions:
(P) Standards of patent and proprietary medicines
(Q) Requirements and guidelines for clinical trials
(R) Standards for sterilized umbilical tapes
(S) Standards for Ophthalmic preparations- GPAT - 2025
- Drug therapy
- If the label or the container bears the name of an individual or company purporting to be the manufacturer of the drug, which individual or company is fictitious or does not exist, it is:
- GPAT - 2025
- Drug therapy
- Manufacturing Specification for tooling has been standardized by?
- GPAT - 2025
- Pharmacy Profession & Introduction to Pharmaceuticals
- As per USP, the maximum concentration of benzalkonium chloride used as a preservative in parenteral formulations is
- GPAT - 2025
- Pharmaceutical Analysis
Match the following:
(P) Schedule H
(Q) Schedule G
(R) Schedule P
(S) Schedule F2
Descriptions:
(I) Life period of drugs
(II) Drugs used under RMP
(III) List of Prescription Drugs
(IV) Standards for surgical dressing
- GPAT - 2025
- Drug therapy
View More Questions