Question:
A thermodynamic system is taken through the cycle abcda. The work done by the gas along the path bc is :
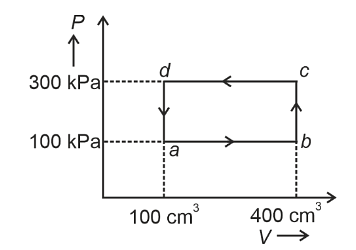
A thermodynamic system is taken through the cycle abcda. The work done by the gas along the path bc is :


Updated On: Jan 13, 2026
- Zero
- 30 J
- –90 J
- –60 J
Hide Solution
Verified By Collegedunia
The Correct Option is A
Solution and Explanation
Understanding the Concept
Work done by a gas in a thermodynamic process is given by the integral of pressure with respect to volume:
\[ W = \int P \, dV \]
In simpler terms, on a P-V diagram, the work done is represented by the area under the curve for the given process.
Analyzing Path bc
Looking at the P-V diagram, path bc is a vertical line. This means the volume remains constant during this process. Such a process is called an isochoric or isovolumetric process.
Since the volume doesn't change (dV = 0), the work done along this path is:
\[ W = \int P \, dV = 0 \]
Therefore, the work done by the gas along the path bc is 0.
Was this answer helpful?
3
3
Top Questions on Thermodynamics
- A weak acid HA has degree of dissociation x. Which option gives the correct expression of \(pH - pK_a\)?
- JEE Main - 2025
- Chemistry
- Thermodynamics
- The molecules having square pyramidal geometry are:
- JEE Main - 2025
- Chemistry
- Thermodynamics
- The standard reduction potential values of some of the p-block ions are given below. Predict the one with the strongest oxidising capacity.
- JEE Main - 2025
- Chemistry
- Thermodynamics
- The incorrect decreasing order of atomic radii is:
- JEE Main - 2025
- Chemistry
- Thermodynamics
- What is the freezing point depression constant of a solvent, 50 g of which contain 1 g non-volatile solute (molar mass 256 g mol\(^{-1}\)) and the decrease in freezing point is 0.40 K?
- JEE Main - 2025
- Chemistry
- Thermodynamics
View More Questions
Questions Asked in NEET exam
- Two cities X and Y are connected by a regular bus service with a bus leaving in either direction every T min. A girl is driving scooty with a speed of 60 km/h in the direction X to Y. She notices that a bus goes past her every 30 minutes in the direction of her motion, and every 10 minutes in the opposite direction. Choose the correct option for the period T of the bus service and the speed (assumed constant) of the buses.
- NEET (UG) - 2025
- Relative Velocity
- A physical quantity P is related to four observations a, b, c, and d as follows: P = a3 b2 (c / √d) The percentage errors of measurement in a, b, c, and d are 1%, 3%, 2%, and 4% respectively. The percentage error in the quantity P is:
- NEET (UG) - 2025
- Dimensional analysis and its applications
What is Microalbuminuria ?
- NEET (UG) - 2025
- Human physiology
The output (Y) of the given logic implementation is similar to the output of an/a …………. gate.

- NEET (UG) - 2025
- Logic gates
- An oxygen cylinder of volume 30 litre has 18.20 moles of oxygen. After some oxygen is withdrawn from the cylinder, its gauge pressure drops to 11 atmospheric pressure at temperature \(27^\circ\)C. The mass of the oxygen withdrawn from the cylinder is nearly equal to: [Given, \(R = \frac{100}{12} \text{ J mol}^{-1} \text{K}^{-1}\), and molecular mass of \(O_2 = 32 \text{ g/mol}\), 1 atm pressure = \(1.01 \times 10^5 \text{ N/m}^2\)]
- NEET (UG) - 2025
- Ideal-gas equation and absolute temperature
View More Questions