Which of the following liquid pairs shows a positive deviation from Raoult's law?
Which of the following liquid pairs shows a positive deviation from Raoult's law?
- Water - hydrochloric acid
- Acetone - chloroform
- Water - nitric acid
- Benzene - methanol
The Correct Option is D
Approach Solution - 1
A positive deviation from Raoult's law occurs when the vapor pressure of a solution is higher than what would be expected based on the ideal behavior predicted by Raoult's law. The vapor pressure of each component is directly proportional to its mole fraction in the solution.
Benzene in methanol breaks the H - bonding of the alcohol making its boiling point decrease & there by its vapour pressure increases leading two +ve deviation.
Causes of positive deviation from Raoult’s law
- Intermolecular forces
- Molecular size and shape
- Hydrogen bonding
Read more from chapter: Solutions
Approach Solution -2
The Correct Answer is (D)
Real Life Applications
- Paints and varnishes: Benzene and methanol are often used in the production of paints and varnishes because they have a high vapor pressure. This means that they evaporate quickly, which allows the paint or varnish to dry quickly.

- Solvents: Acetone and chloroform are used as solvents because they have a high vapor pressure. This means that they can dissolve other substances easily.
- Cloud formation: The formation of clouds is thought to be due to the positive deviation from Raoult's law of water vapor and air. This means that the vapor pressure of water vapor in the air is greater than what would be expected according to Raoult's law. This extra vapor pressure causes the water vapor to condense into clouds.
Question can also be asked as
- Which of the following liquid pairs shows an increase in vapor pressure above what is predicted by Raoult's law?
- Which of the following liquid pairs shows an increase in the total vapor pressure above what is predicted by Raoult's law?
- Which of the following liquid pairs shows a decrease in the intermolecular forces of attraction between the molecules?
Approach Solution -3
Solution is a mixture of a solute into a solvent. The solution can be homogenous and heterogenous, based on the size of the particle. Some of the common examples of solutions are Sugar and salt in water solutions, soda water. A solution can be formed by gases and solid also.
A solution has two components:
- Solute
- Solvent
The concentration of the solution is based on the proportion of the solute in the solvent. It can be of three types:
- Diluted
- Concentrated
- Saturated
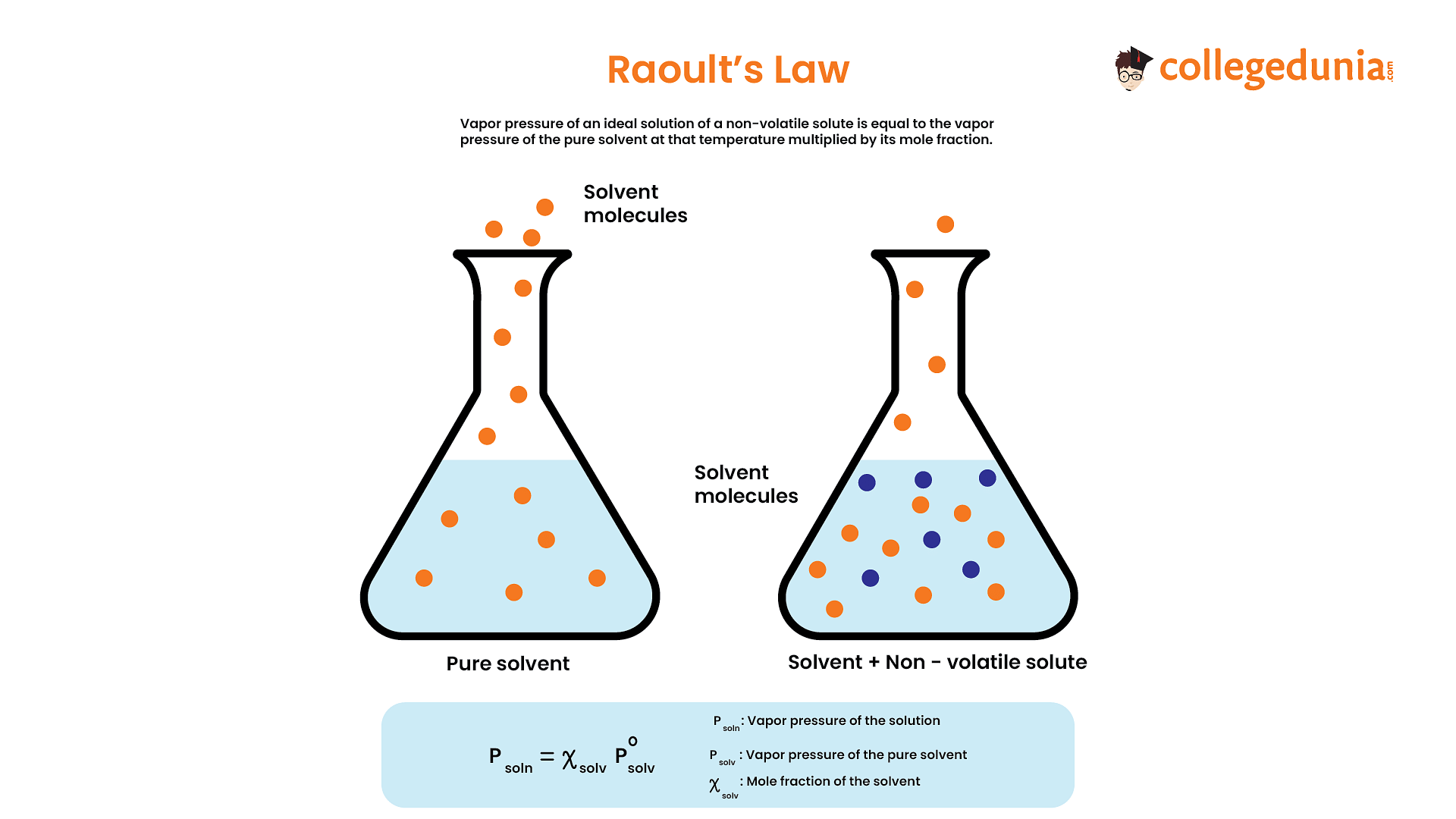
Raoult’s Law
This law is applicable for the two volatile liquids. According to this law, the partial pressure of the component in the solution is directly proportional to the mole fraction of the component.
P= P₀X
Where,
- P0 = Vapour pressure of pure liquid component 1 at the same temperature
- Χ = Mole fraction of component in solution.
- P = Vapour pressure of component in solution.
There can be both positive and negative deviation from Raoult’s law:
Positive deviation: A positive deviation from Raoult's law occurs when the vapor pressure of a solution is higher than what would be expected based on the ideal behavior predicted by Raoult's law. Eg: Water and ethanol,
Negative deviation: A negative deviation from Raoult's law occurs when the vapor pressure of a solution is lower than what would be expected based on the ideal behavior predicted by Raoult's law. Eg: a mixture of acetone and chloroform
Read more:
| Related Concepts | ||
|---|---|---|
| Dalton's law of Partial pressure | Light | Hypertonic solution |
| Alloys | Fluid | Osmotic pressure |
Learn with videos:
Top Questions on Solutions
- Consider the dissociation equilibrium of the following weak acid: \[ \mathrm{HA \rightleftharpoons H^+(aq) + A^-(aq)} \] If the \(pK_a\) of the acid is \(4\), then the pH of a \(10\ \text{mM}\) HA solution is ________ (Nearest integer). (Given: The degree of dissociation can be neglected with respect to unity)
- The crystal field splitting energy of $[Co(oxalate)_3]^3-$ complex is 'n' times that of the $[Cr(oxalate)_3]^3-$ complex. Here 'n' is_______ (Assume $\Delta_0 > P$)}
- The osmotic pressure of a living cell is 12 atm at 300 K. The strength of sodium chloride solution that is isotonic with the living cell at this temperature is ____________ g L$^{-1}$. (Nearest integer)
Given: R = 0.08 L atm K$^{-1}$ mol$^{-1}$
Assume complete dissociation of NaCl
(Given : Molar mass of Na and Cl are 23 and 35.5 g mol$^{-1}$ respectively.) A substance 'X' (1.5 g) dissolved in 150 g of a solvent 'Y' (molar mass = 300 g mol$^{-1}$) led to an elevation of the boiling point by 0.5 K. The relative lowering in the vapour pressure of the solvent 'Y' is $____________ \(\times 10^{-2}\). (nearest integer)
[Given : $K_{b}$ of the solvent = 5.0 K kg mol$^{-1}$]
Assume the solution to be dilute and no association or dissociation of X takes place in solution.- At \(T\) K, \(100\,\text{g}\) of \(98%\) \(H_2SO_4\) (w/w) aqueous solution is mixed with \(100\,\text{g}\) of \(49%\) \(H_2SO_4\) (w/w) aqueous solution. What is the mole fraction of \(H_2SO_4\) in the resultant solution? (Given: Atomic mass \(H = 1\,u,\; S = 32\,u,\; O = 16\,u\). Assume that temperature after mixing remains constant.)
Questions Asked in AIEEE exam
- A steel wire can sustain $100\, kg$ weight without breaking. If the wire is cut into two equal parts, each part can sustain a weight of
- AIEEE - 2012
- mechanical properties of solids
- If the line $y = mx + 1$ meets the circle $x^2 + y^2 + 3x = 0 $ in two points equidistant from and on opposite sides of $x$-axis, then
- AIEEE - 2012
- Conic sections
- This question has Statement 1 and Statement 2. Of the four choices given after the Statements, choose the one that best describes the two Statements. If you push on a cart being pulled by a horse so that it does not move, the cart pushes you back with an equal and opposite force. The cart does not move because the force described in statement 1 cancel each other.
- AIEEE - 2012
- potential energy
- The variance of first n odd natural numbers is $\frac{n^{2}-1}{3}$ : The sum of first n odd natural number is $n^2$ and the sum of square of first n odd natural numbers is $\frac{n\left(4n^{2}-1\right)}{3}.$
- AIEEE - 2012
- Variance and Standard Deviation
- The ratio of number of oxygen atoms (O) in 16.0 g ozone $(O_3), \,28.0\, g$ carbon monoxide $(CO)$ and $16.0$ oxygen $(O_2)$ is (Atomic mass: $C = 12,0 = 16$ and Avogadro?? constant $N_A = 6.0 x 10^{23}\, mol^{-1}$)
- AIEEE - 2012
- Mole concept and Molar Masses
Concepts Used:
Solutions
A solution is a homogeneous mixture of two or more components in which the particle size is smaller than 1 nm.
For example, salt and sugar is a good illustration of a solution. A solution can be categorized into several components.
Types of Solutions:
The solutions can be classified into three types:
- Solid Solutions - In these solutions, the solvent is in a Solid-state.
- Liquid Solutions- In these solutions, the solvent is in a Liquid state.
- Gaseous Solutions - In these solutions, the solvent is in a Gaseous state.
On the basis of the amount of solute dissolved in a solvent, solutions are divided into the following types:
- Unsaturated Solution- A solution in which more solute can be dissolved without raising the temperature of the solution is known as an unsaturated solution.
- Saturated Solution- A solution in which no solute can be dissolved after reaching a certain amount of temperature is known as an unsaturated saturated solution.
- Supersaturated Solution- A solution that contains more solute than the maximum amount at a certain temperature is known as a supersaturated solution.
