What is the pressure of 2 mole of $NH_3$ at 27$^\circ$C when its volume is 5 lit. in van der Waals equation (a = 4.17, b = 0 .03711)
- 10.33 aun
- 9.333 atm.
- 9.74 atm
- 9.2 atm
The Correct Option is B
Solution and Explanation
R = 0.082 L atm $K^{-1} \, mol^{-1}$
n = 2 mol, a = 4.17
V = 5 L b = 0.03711
$\left(P + \frac{an^2}{V^2} \right) $ (V - nb) = nRT
$\left(P + \frac{4.17 \times 2^2}{5^2} \right) (5 -2 \times 0.3711) = 2 \times 0.0821 \times 300 $
$\left(P + \frac{4.17 \times 4}{25} \right) (5 - 0.07422) = 600 \times 0.0821$
(P + 0.67) (4-93) = 600 $\times$ 0.0821
P + 0.67 = $\frac{600 \times 0.0821}{4.93}$
P + 0.67 = 9.99
P = 9.99 - 0.67 = 9.32 atm
Top Questions on Van Der Waals equation
- Molar volume ($ V_m $) of a van der Waals gas can be calculated by expressing the van der Waals equation as a cubic equation with $ V_m $ as the variable. The ratio (in mol dm$^{-3}$) of the coefficient of $ V_m^2 $ to the coefficient of $ V_m $ for a gas having van der Waals constants $ a = 6.0 \, \text{dm}^6 \, \text{atm mol}^{-2} $ and $ b = 0.060 \, \text{dm}^3 \, \text{mol}^{-1} $ at 300 K and 300 atm is ____. Use: Universal gas constant $ R = 0.082 \, \text{dm}^3 \, \text{atm mol}^{-1} \, \text{K}^{-1} $
- JEE Advanced - 2025
- Chemistry
- Van Der Waals equation
- Arrange the following gases in increasing order of van der Waals constant 'a'
A. Ar
B. CH4
C. H₂O
D. C6H6
Choose the correct option from the following.- JEE Main - 2023
- Chemistry
- Van Der Waals equation
- Which of the following statemnt is incorect for physisorption?
- GUJCET - 2023
- Chemistry
- Van Der Waals equation
- At low pressure, the van der Waal's equation is reduced to
- VITEEE - 2019
- Chemistry
- Van Der Waals equation
- If $V$ is the volume of one molecule of gas under given conditions, the van der Waal?s constant $b$ is
- BITSAT - 2018
- Chemistry
- Van Der Waals equation
Questions Asked in OJEE exam
Consider the following statements: Statement I: \( 5 + 8 = 12 \) or 11 is a prime. Statement II: Sun is a planet or 9 is a prime.
Which of the following is true?
- OJEE - 2025
- Number System
The value of \[ \int \sin(\log x) \, dx + \int \cos(\log x) \, dx \] is equal to
- OJEE - 2025
- Calculus
The value of \[ \lim_{x \to \infty} \left( e^x + e^{-x} - e^x \right) \] is equal to
- OJEE - 2025
- Calculus
- Consider the function
\[ f(x) = \begin{cases} \frac{\log(1+3x) - \log(1-2x)}{x}, & x \neq 0 \\ k, & x = 0 \end{cases} \] If \( f \) is continuous at \( x = 0 \), then the value of \( k \) must be
- OJEE - 2025
- Calculus
- Pick out the most effective word(s) from the given words to fill in the blank to make the sentence meaningfully complete.
The miser gazed ...... at the pile of gold coins in front of him.
- OJEE - 2025
- Grammar
Concepts Used:
Van Der Waals Equation
Van der Waals equation is an equation relating the relationship between the pressure, volume, temperature, and amount of real gases.
Read More: Derivation of Van Der Waals Equation
Derivation of Van der Waals equation:
For a real gas containing ‘n’ moles, the equation is written as
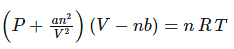
Where, P, V, T, n are the pressure, volume, temperature and moles of the gas. ‘a’ and ‘b’ constants specific to each gas.
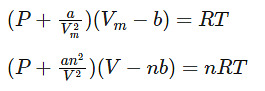
Where,
Vm: molar volume of the gas
R: universal gas constant
T: temperature
P: pressure
V: volume
Thus, Van der Waals equation can be reduced to ideal gas law as PVm = RT.
The equation can further be written as;
- Cube power of volume:
- Reduced equation (Law of corresponding states) in terms of critical constants:
Units of Van der Waals equation Constants
a: atm lit² mol-²
b: litre mol-¹