The total number of head to tail isoprene linkages in the following molecule is ..........

The total number of head to tail isoprene linkages in the following molecule is .......... 
Show Hint
Correct Answer: 4
Solution and Explanation
To determine the number of head-to-tail isoprene linkages in the given molecule, we first need to understand the structure of an isoprene unit, which is 2-methyl-1,3-butadiene. The "head" is the branched end (methyl group), and the "tail" is the end opposite the methyl group.
1. Identify Isoprene Units: Observe the repeating units consisting of carbon backbones with attached methyl branches.
2. Map Head-to-Tail Linkages:
- Consider each unit as an isoprene segment.
- Check for the connection points where the "tail" of one isoprene unit connects to the "head" of the next unit.
By examining the structure, it's evident that the linkages are as follows:
- First unit to second unit
- Second unit to third unit
- Third unit to fourth unit
- Fourth unit to fifth unit
3. Count Linkages: There are 4 head-to-tail linkages in total.
Thus, the total number of head-to-tail isoprene linkages in the molecule is 4.
Top Questions on Natural Products Chemistry
- The pair of proteins having heme core is
- IIT JAM CY - 2024
- Organic Chemistry
- Natural Products Chemistry
- The following dipeptide derivative is used as an artificial sweetener:
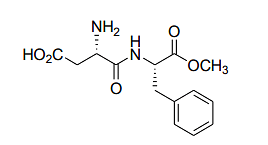
The constituent α-amino acids of this dipeptide are- IIT JAM CY - 2024
- Organic Chemistry
- Natural Products Chemistry
Questions Asked in IIT JAM CY exam
- Among the following, the correct condition(s) for spontaneity is(are)
- IIT JAM CY - 2025
- Thermodynamics
One mole of a monoatomic ideal gas starting from state A, goes through B and C to state D, as shown in the figure. Total change in entropy (in J K\(^{-1}\)) during this process is ...............

- IIT JAM CY - 2025
- Thermodynamics
The number of chiral carbon centers in the following molecule is ...............

- IIT JAM CY - 2025
- General Chemistry
- Consider the following matrices A and B.
\[ A = \begin{pmatrix} 1 & 2 & 0 & 0 \\ 3 & 4 & 0 & 0 \\ 0 & 5 & 5 & 0 \\ 0 & 0 & 6 & 7 \\ 0 & 0 & 8 & 9 \end{pmatrix} \quad \text{and} \quad B = \begin{pmatrix} 10 & 11 & 0 & 0 & 0 \\ 12 & 13 & 0 & 0 & 0 \\ 0 & 0 & 4 & 0 & 0 \\ 0 & 0 & 15 & 16 & 0 \\ 0 & 0 & 17 & 18 & 0 \end{pmatrix} \]
If \( C = AB \), the sum of the diagonal elements of \( C \) is ..............
- IIT JAM CY - 2025
- General Chemistry
A tube fitted with a semipermeable membrane is dipped into 0.001 M NaCl solution at 300 K as shown in the figure. Assume density of the solvent and solution are the same. At equilibrium, the height of the liquid column \( h \) (in cm) is .........

- IIT JAM CY - 2025
- General Chemistry