Question:
The following set of reactions are used in refining zirconium.
$Zr (impure) + 2I_2$ ${->[523K]}$ $ZrI_4$ ${->[1800K]}$ Zr (pure) +$2I_2$
This method is known as
The following set of reactions are used in refining zirconium.
$Zr (impure) + 2I_2$ ${->[523K]}$ $ZrI_4$ ${->[1800K]}$ Zr (pure) +$2I_2$
This method is known as
Updated On: Jun 6, 2022
- Distillation
- Liquation
- Hall-Heroult method
- Van Arkel method
Hide Solution
Verified By Collegedunia
The Correct Option is D
Solution and Explanation
Answer (d) Van Arkel method
Was this answer helpful?
0
0
Top Questions on General Principles and Processes of Isolation of Elements
- What is used for the Thermite Reaction?
- BCECE - 2025
- Chemistry
- General Principles and Processes of Isolation of Elements
- In the extraction of iron using blast furnace to remove the impurity (X), chemical (Y) is added to the ore. X and Y are respectively
- TS EAMCET - 2025
- Chemistry
- General Principles and Processes of Isolation of Elements
- Which of the following compounds is used to cover the surface of a metallic object to prevent corrosion?
- KEAM - 2025
- Chemistry
- General Principles and Processes of Isolation of Elements
- The incorrect statement about the Hall-Heroult process is:
- KCET - 2024
- Chemistry
- General Principles and Processes of Isolation of Elements
- Select the correct statement:
- KCET - 2024
- Chemistry
- General Principles and Processes of Isolation of Elements
View More Questions
Questions Asked in KEAM exam
- Which among the following has the highest molar elevation constant?
- KEAM - 2025
- Colligative Properties
- The formula of Ammonium phosphomolybdate is
- KEAM - 2025
- coordination compounds
- Which is a Lewis acid?
- KEAM - 2025
- Acids and Bases
- Hardness of water is estimated by titration with
- KEAM - 2025
- Solutions
- Which of the following gases has the lowest solubility in water at 298 K?
- KEAM - 2025
- Solutions
View More Questions
Concepts Used:
General Principles and Processes of Isolation of Elements
What are Ores and Minerals?
Minerals are the naturally occurring, homogeneous inorganic solid substances. They are having a definite chemical composition and crystalline structure, hardness and color. For example, copper pyrite, calamine, etc.
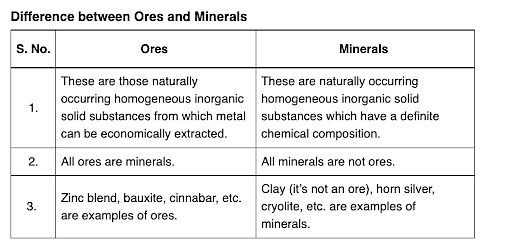
Impurities in an ore are called gauge. The removal of a gauge from the ore is called concentration ore.
Several steps are involved in the extraction of pure metal from ores. Major steps are as follows –
- Concentration of the ore
- Isolation of the metal from its concentrated ore
- Purification of the metal