The solubility of MX2–type electrolytes is 0.5 × 10–4 Mole/lit. then find out the Ksp of electrolytes :
The solubility of MX2–type electrolytes is 0.5 × 10–4 Mole/lit. then find out the Ksp of electrolytes :
5 × 10–12
25 × 10–10
1 × 10–13
5 × 10–13
The Correct Option is D
Solution and Explanation
The correct option is (D) : 5 × 10–13
An electrolyte MX2 undergoes dissociation as follows :
MX2\(\rightleftharpoons\)M+2+2X− Concentration MX2 M+2 X− Initial concentration 1 0 0 Concentration at Equilibrium 1-s s 2s Thus from the above condition we can say that, Ksp=s×(2s)2=4×(s)3 Here, s (the solubility ) is 0.5×10−4mole/lit. ∴ Ksp =4×(0.5×10−4)3 ∴ Ksp=5×10−13
Top Questions on Equilibrium
Consider the following equilibrium,
CO(g) + 2H2(g) ↔ CH3OH(g)
0.1 mol of CO along with a catalyst is present in a 2 dm3 flask maintained at 500 K. Hydrogen is introduced into the flask until the pressure is 5 bar and 0.04 mol of CH3OH is formed. The Kp is ____ × 10-3 (nearest integer).
Given: R = 0.08 dm3 bar K-1mol-1
Assume only methanol is formed as the product and the system follows ideal gas behaviour.
- JEE Main - 2025
- Chemistry
- Equilibrium
The pH of a 0.01 M weak acid $\mathrm{HX}\left(\mathrm{K}_{\mathrm{a}}=4 \times 10^{-10}\right)$ is found to be 5 . Now the acid solution is diluted with excess of water so that the pH of the solution changes to 6 . The new concentration of the diluted weak acid is given as $\mathrm{x} \times 10^{-4} \mathrm{M}$. The value of x is _______ (nearest integer).
- JEE Main - 2025
- Chemistry
- Equilibrium
A body of mass $m$ is suspended by two strings making angles $\theta_{1}$ and $\theta_{2}$ with the horizontal ceiling with tensions $\mathrm{T}_{1}$ and $\mathrm{T}_{2}$ simultaneously. $\mathrm{T}_{1}$ and $\mathrm{T}_{2}$ are related by $\mathrm{T}_{1}=\sqrt{3} \mathrm{~T}_{2}$. the angles $\theta_{1}$ and $\theta_{2}$ are
- JEE Main - 2025
- Physics
- Equilibrium
- According to Le Chatelier's principle, in the reaction \( \text{CO}(g) + 3\text{H}_2(g) \rightleftharpoons \text{CH}_4(g) + \text{H}_2\text{O}(g) \), the formation of methane is favoured by
- KCET - 2025
- Chemistry
- Equilibrium
- Which of the following statements is/are true about equilibrium?
- KCET - 2025
- Chemistry
- Equilibrium
Questions Asked in NEET exam
- Two cities X and Y are connected by a regular bus service with a bus leaving in either direction every T min. A girl is driving scooty with a speed of 60 km/h in the direction X to Y. She notices that a bus goes past her every 30 minutes in the direction of her motion, and every 10 minutes in the opposite direction. Choose the correct option for the period T of the bus service and the speed (assumed constant) of the buses.
- NEET (UG) - 2025
- Relative Velocity
- A physical quantity P is related to four observations a, b, c, and d as follows: P = a3 b2 (c / √d) The percentage errors of measurement in a, b, c, and d are 1%, 3%, 2%, and 4% respectively. The percentage error in the quantity P is:
- NEET (UG) - 2025
- Dimensional analysis and its applications
What is Microalbuminuria ?
- NEET (UG) - 2025
- Human physiology
The output (Y) of the given logic implementation is similar to the output of an/a …………. gate.

- NEET (UG) - 2025
- Logic gates
- An oxygen cylinder of volume 30 litre has 18.20 moles of oxygen. After some oxygen is withdrawn from the cylinder, its gauge pressure drops to 11 atmospheric pressure at temperature \(27^\circ\)C. The mass of the oxygen withdrawn from the cylinder is nearly equal to: [Given, \(R = \frac{100}{12} \text{ J mol}^{-1} \text{K}^{-1}\), and molecular mass of \(O_2 = 32 \text{ g/mol}\), 1 atm pressure = \(1.01 \times 10^5 \text{ N/m}^2\)]
- NEET (UG) - 2025
- Ideal-gas equation and absolute temperature
Concepts Used:
Law of Chemical Equilibrium
Law of Chemical Equilibrium states that at a constant temperature, the rate of a chemical reaction is directly proportional to the product of the molar concentrations of the reactants each raised to a power equal to the corresponding stoichiometric coefficients as represented by the balanced chemical equation.
Let us consider a general reversible reaction;
A+B ↔ C+D
After some time, there is a reduction in reactants A and B and an accumulation of the products C and D. As a result, the rate of the forward reaction decreases and that of backward reaction increases.
Eventually, the two reactions occur at the same rate and a state of equilibrium is attained.
By applying the Law of Mass Action;
The rate of forward reaction;
Rf = Kf [A]a [B]b
The rate of backward reaction;
Rb = Kb [C]c [D]d
Where,
[A], [B], [C] and [D] are the concentrations of A, B, C and D at equilibrium respectively.
a, b, c, and d are the stoichiometric coefficients of A, B, C and D respectively.
Kf and Kb are the rate constants of forward and backward reactions.
However, at equilibrium,
Rate of forward reaction = Rate of backward reaction.
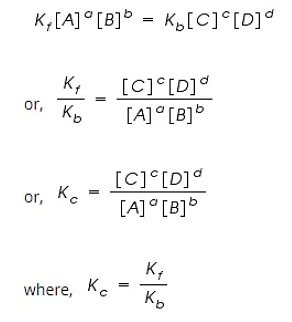
Kc is called the equilibrium constant expressed in terms of molar concentrations.
The above equation is known as the equation of Law of Chemical Equilibrium.