Name the products of the following reactions.
(I) $C_6H_6$ reacts with methyl chloride in presence of $AlCl_3$.
(II) $C_6H_6$ reacts with acetyl chloride in presence of $AlCl_3$.
(III) $C_6H_6$ reacts with fuming nitric acid in presence of cone. $H_2SO_4$.
(IV) $C_6H_6$ is catalytically hydrogenated.
- a
- b
- c
- d
The Correct Option is D
Solution and Explanation
 undefined
undefined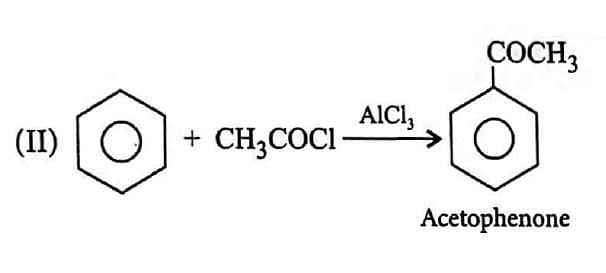 undefined
undefined undefined
undefined
Top Questions on Aromatic Hydrocarbon
Which of the following are aromatic?

- CUET (PG) - 2025
- Chemistry
- Aromatic Hydrocarbon
- When C6H5CHO reacts with the mixture of HNO3 and H2SO4 at 273-283K, it gives
- KEAM - 2025
- Chemistry
- Aromatic Hydrocarbon
- The temperature and pressure required for reforming benzene from n-hexane is
- KEAM - 2025
- Chemistry
- Aromatic Hydrocarbon
- Which of the following is non-aromatic?
- KEAM - 2025
- Chemistry
- Aromatic Hydrocarbon
Give plausible explanation for:
(a) Diazonium salts of aromatic amines are stable.
(b) Aniline does not undergo Friedel-Crafts reaction.
(c) Aniline on nitration gives substantial meta product.- CBSE CLASS XII - 2024
- Chemistry
- Aromatic Hydrocarbon
Concepts Used:
Aromatic hydrocarbon
Aromatic hydrocarbons, sometimes known as arenes, are aromatic organic molecules made up entirely of carbon and hydrogen. In aromatic compounds a benzene ring which is named after the simple aromatic chemical benzene, or a phenyl group when part of a larger structure, is the configuration of six carbon atoms.
Read More: Aromaticity
Reactions of Aromatic Hydrocarbons:
1. Aromatic Substitution Reactions
This reaction involves the replacement of one substituent on the ring of an aromatic hydrocarbon, commonly a hydrogen atom, by a different substituent group.
The common types of aromatic substitution reactions are:
- Nucleophilic aromatic substitution reactions
- Electrophilic aromatic substitution reactions
- Radical nucleophilic aromatic substitution reactions
2. Coupling Reactions
In these types of reactions, the coupling of two fragments that have a radical nature is achieved with the help of a metal catalyst